Non-small cell lung cancer: the global clinical trials landscape 2024
Pharmaceutical Technology
JULY 16, 2024
Accounting for around 85% of lung cancer cases worldwide, non-small cell lung cancer is a key research area for clinical trials in 2024.

Pharmaceutical Technology
JULY 16, 2024
Accounting for around 85% of lung cancer cases worldwide, non-small cell lung cancer is a key research area for clinical trials in 2024.

Bio Pharma Dive
JULY 16, 2024
The well-funded startup says drugs AbbVie abandoned last year could form a combination regimen with “superior efficacy” to Vertex’s market-leading medicines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 16, 2024
AstraZeneca has closed the acquisition of Amolyt Pharma, a company specialising in treatments for rare endocrine diseases.
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 16, 2024
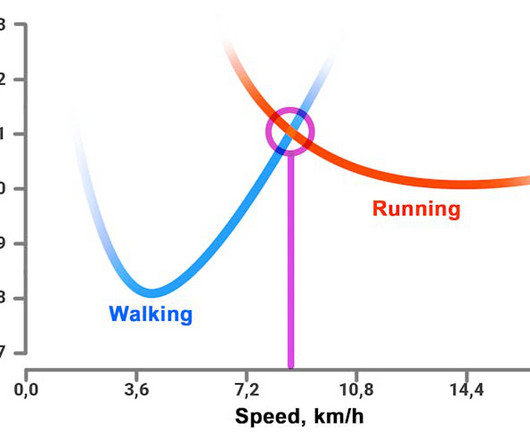
It’s Monday morning, the alarm goes off and it’s already 7:30 a.m. – and you’re 30 minutes late. Normally you need 45 minutes to walk the 3 kilometres to work, but this morning you’ll be running for 20 minutes.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Pharmaceutical Technology
JULY 16, 2024
Kyverna’s CEO Peter Maag said the biotech is “eager to start generating data” from a Phase II trial.

Bio Pharma Dive
JULY 16, 2024
In the suit, Vertex argues HHS’ stance forces a “Hobson’s choice” on people with sickle cell seeking to undergo the potentially curative treatment.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
JULY 16, 2024
The Merck KGaA spinout has a tau-regulating medicine it claims could be an “ideal therapy” for Alzheimer’s. The pitch has intrigued both healthcare investors and pharma venture arms.
Pharma Times
JULY 16, 2024
The progressive neurodegenerative condition affects around 153,000 people in the UK

Bio Pharma Dive
JULY 16, 2024
The financing, which ranks as one of the year’s larger private rounds, will help the startup advance a pair of drugs and potentially acquire other medicines.

Rethinking Clinical Trials
JULY 16, 2024
A recent report from the Assistant Secretary for Planning and Evaluation in the US Department of Health and Human Services outlines 36 projects funded by the Office of the Secretary Patient-Centered Outcomes Research Trust Fund (OS-PCORTF). OS-PCORTF was created to build national data capacity and infrastructure and leverage existing clinical data and federal data for patient-centered outcomes research.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
JULY 16, 2024
The deal gives Vertex rights to use Orum’s ADC-like technology to discover gentler preparatory regimens for patients receiving intensive treatments like Casgevy.
Pharma Times
JULY 16, 2024
Myelodysplastic syndromes are a group of blood cancers that currently affect more than 7,000 people in the UK

Pharmaceutical Technology
JULY 16, 2024
The Phase III RASolute 302 trial will compare a 300mg dose of the company’s investigational pan-RAS inhibitor RMC-6236 to chemotherapy.

BioSpace
JULY 16, 2024
Follow along as BioSpace keeps you up-to-date on the latest pharma and biotech layoffs.
Pharmaceutical Technology
JULY 16, 2024
SOTIO will pay up to $325.5m in upfront and milestone payments for developing multiple bispecific antibody drug conjugates (ADCs).

pharmaphorum
JULY 16, 2024
Novo Holdings leads $100m financing for Swiss biotech Asceneuron, which is developing an oral tau therapy for Alzheimer's disease

Pharmaceutical Technology
JULY 16, 2024
Asceneuron has announced an oversubscribed Series C financing round, securing $100m, for advancing neurodegenerative therapeutics.

pharmaphorum
JULY 16, 2024
A digital diagnostic for ADHD called QbTest should be used by the NHS as an add-on to standard assessment, says new NICE guidance

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
JULY 16, 2024
The US Food and Drug Administration (FDA) has granted fast track designation to Sumitomo Pharma America’s (SMPA) DSP-5336 for AML.

pharmaphorum
JULY 16, 2024
CordenPharma has launched a €900 million ($981 million) investment programme to prepare for an expected surge in demand for peptide production capacity – driven by GLP-1 receptor agonists.The three-year plan revolves around parallel programmes in the US and Europe that will include new builds and upgrades and expansion to its existing facilities, according to the German contract development and manufacturing organisation (CDMO).
Pharmaceutical Technology
JULY 16, 2024
The R21/Matrix-M malaria vaccine, co-developed by SII and the University of Oxford, has been launched in Côte d'Ivoire

pharmaphorum
JULY 16, 2024
Natural killer cell therapy specialist Artiva Biotherapeutics has another go at an IPO, hoping to raise up to $135m

Fierce Pharma
JULY 16, 2024
Despite a string of high-profile losses this year, at least three major drugmakers continue to fight back against the Medicare price negotiations being rolled out under the Inflation Reduction Act. | Despite a string of high-profile losses this year, at least three major drugmakers continue to fight back against the Medicare price negotiations being rolled out under the Inflation Reduction Act.

Drug Patent Watch
JULY 16, 2024
Introduction Patent oppositions are a crucial tool in the pharmaceutical industry, allowing civil society to challenge the validity of patents […] Source

Outsourcing Pharma
JULY 16, 2024
OMNY Health is changing the healthcare industry by connecting providers and life sciences companies through a comprehensive data ecosystem.

pharmaphorum
JULY 16, 2024
The pandemic has exposed vulnerabilities in global supply chains, particularly in the healthcare sector, concerning the production and distribution of essential medical supplies, such as antibiotics and other medications. Learn more about the impact and potential solutions.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

BioPharma Reporter
JULY 16, 2024
Integrated DNA Technologies (IDT) is doubling down on its commitment to innovation with the launch of a new 25,000-square-foot synthetic biology facility in Coralville, Iowa.

Pharmaceutical Commerce
JULY 16, 2024
The latest news for pharma industry insiders.

Fierce Pharma
JULY 16, 2024
As Johnson & Johnson continues to attempt to Texas two-step its way around thousands of claims that its popular talcum powder-based products caused cancer, the drugmaker is putting up hundreds | As Johnson & Johson continues to attempt to Texas two-step its way around thousands of claims that its popular talcum powder-based products caused cancer, the drugmaker is putting up hundreds of millions of dollars to settle a longstanding dispute with a clutch of suppliers.

BioSpace
JULY 16, 2024
As scrutiny on pharmacy benefit managers mount, a House committee will hold a hearing on the alleged anticompetitive business practices of these middlemen.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content