Cloud Computing in the Pharmaceutical Industry
Pharmaceutical Technology
OCTOBER 2, 2024
Discover the leading providers of cloud computing in the pharmaceutical industry. Download the free Buyer's Guide for full contact details.
Pharmaceutical Technology
OCTOBER 2, 2024
Discover the leading providers of cloud computing in the pharmaceutical industry. Download the free Buyer's Guide for full contact details.

Bio Pharma Dive
OCTOBER 2, 2024
Biosimilar competition to aging blockbusters will erode a large chunk of the pharma giant’s top line over the next few years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 2, 2024
Exscientia detailed how its robotics lab in Oxfordshire, UK is increasing productivity and efficiency in developing new molecules at ELRIG 2024.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 2, 2024
(akaratwimages/Canva) High blood pressure (or hypertension) increases the risk of health problems such as heart or kidney failure, but goes undiagnosed in millions of people.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!
Pharmaceutical Technology
OCTOBER 2, 2024
The funds will support the development of LoQus23’s lead candidate, its MutSβ inhibitor, as it enters clinical development.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 2, 2024
The Jan Aushadhi Kendras (JAKs) under the flagship scheme of the Department of Pharmaceuticals (DoP), Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP), has reported a growth of 6.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharma Times
OCTOBER 2, 2024
ISM001-055 shows promise in improving lung function among patients

Bio Pharma Dive
OCTOBER 2, 2024
The company will permit six generic drugmakers to make and sell lenacapavir in 120 countries that have high incidence of the disease but limited resources.

Rethinking Clinical Trials
OCTOBER 2, 2024
In this Friday’s PCT Grand Rounds, Tyler Winkelman of Hennepin Healthcare and the Hennepin Healthcare Research Institute, and David Johnson of Hennepin County, will present “Health Trends Across Communities – A Novel Health System-Public Health Data Partnership.” The Grand Rounds session will be held on Friday, October 4, 2024, at 1:00 pm eastern.

Pharma Times
OCTOBER 2, 2024
Preclinical data on NeuroRestore therapy to be showcased at CTAD in Madrid

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
OCTOBER 2, 2024
It’s the first time, according to the insurer, that this type of model has been used to bring a Humira biosimilar to market, and it yields a much lower cost than both the brand-name version of the drug and biologic copycats.

Pharmaceutical Technology
OCTOBER 2, 2024
The Series B funds will expedite pipeline expansion and cover a clinical proof-of-concept study for TRIV-573.

Bio Pharma Dive
OCTOBER 2, 2024
With its latest investment, Lilly is looking toward medicines of the future and new methods to produce them.
Pharmaceutical Technology
OCTOBER 2, 2024
Focus is turning to the development of peptide analogs of antibody-derived radiotherapeutics, according to Dr. Dave Madge.

pharmaphorum
OCTOBER 2, 2024
A federal judge in New York has ruled that Amgen must answer to claims of misleading investors about billions owed to US tax authorities, allowing a securities-fraud class action to proceed against the pharmaceutical giant.

Pharmaceutical Technology
OCTOBER 2, 2024
The cash-saving measures come after interim analysis showed the company’s lead cancer drug only showed modest improvement in survival.

Fierce Pharma
OCTOBER 2, 2024
Gilead Sciences is wasting no time in promoting worldwide access to its twice-yearly pre-exposure prophylaxis (PrEP) HIV candidate lenacapavir. | The company will allow six generic manufacturers to make and sell its long-acting PrEP drug, lenacapivir, in 120 resource-limited countries.

pharmaphorum
OCTOBER 2, 2024
The patient will see you now: Why embracing discomfort could transform patient engagement Mike.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Fierce Pharma
OCTOBER 2, 2024
Aspen Neuroscience, underway with Parkinson's cell therapy trial, bolsters manufacturing with new San Diego facility fkansteiner Wed, 10/02/2024 - 07:31

pharmaphorum
OCTOBER 2, 2024
Explore the mergers, acquisitions, and partnerships in August 2024. Discover how these collaborations are shaping the business landscape.
Drug Patent Watch
OCTOBER 2, 2024
As an outsider, finding out the manufacturing capacity of a given pharmaceutical manufacturing plant can be challenging due to the proprietary nature of such information. However, you can employ several strategies to gather relevant data: Example: Pfizer’s 2022 Annual Report states, “Our manufacturing facilities are located in 39 countries across 6 continents.

pharmaphorum
OCTOBER 2, 2024
FDA issues summary of grants, promotes rare disease research Nicole.
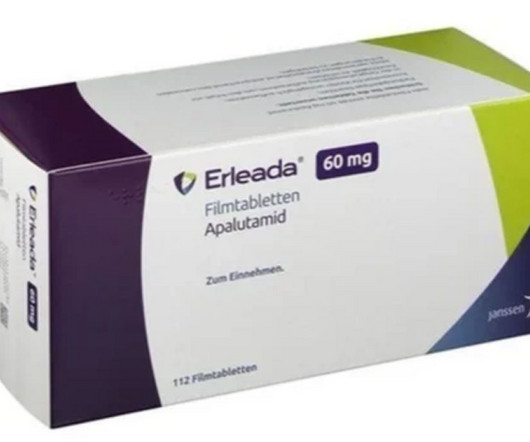
Fierce Pharma
OCTOBER 2, 2024
While they were both approved several years back to treat the same two types of prostate cancer, Astellas and Pfizer’s Xtandi has held a larger slice of the market than Johnson & Johnson’s riva | A real-world, head-to-head study of two androgen receptor pathway inhibiting (ARPI) prostate cancer drugs indicates that Johnson & Johnson’s Erleada provides an edge in overall survival versus Astellas and Pfizer’s Xtandi.

pharmaphorum
OCTOBER 2, 2024
In a bold move to tackle soaring drug costs, Blue Shield of California is pioneering a strategy that bypasses traditional pharmacy benefit managers to slash the cost of Humira by 90%

Fierce Pharma
OCTOBER 2, 2024
Roughly a year and a half after the introduction of a shareholder lawsuit, Amgen has lost its bid to toss the case accusing the California biotech giant of concealing a massive tax bill with the IR | New York District Judge John Cronan has rejected Amgen’s motion to dismiss an investor lawsuit—originally filed by a Michigan-based union pension fund—accusing the company of hiding a $10.7-billion tax bill from the public.

pharmaphorum
OCTOBER 2, 2024
In the VP debate, Tim Walz and JD Vance were forceful, unexpectedly convivial, and fast and loose with the facts, particularly on healthcare.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

CTTI (Clinical Trials Transformation Initiative)
OCTOBER 2, 2024
Implementing less rigid, more flexible approaches to clinical trials can increase patient and site access to research, improve participant satisfaction, facilitate real-world data use, enroll more diverse patient populations, and enable more generalizable trial results. Ultimately, these flexible trial approaches help sponsors bring new therapies to patients faster.

Fierce Pharma
OCTOBER 2, 2024
Sitting down with Emily Keith, SVP at AdTheorent Health, at Digital Pharma East 2024, we explored the cutting-edge int | Sitting down with Emily Keith, SVP at AdTheorent Health, at Digital Pharma East 2024, we explored the cutting-edge intersection of healthcare, advertising and privacy.

FDA Law Blog
OCTOBER 2, 2024
By Adrienne R. Lenz, Principal Medical Device Regulation Expert & Lisa M. Baumhardt, Senior Medical Device Regulation Expert & Gail H. Javitt — A recent draft guidance on predetermined change control plans (PCCP) for medical devices continues FDA’s effort to implement Section 515C of the Food and Drug Omnibus Reform Act of 2022 (FDORA), which Congress enacted to make it easier for manufacturers to make post-market device changes by avoiding the need for additional FDA authorization (se

BioPharma Reporter
OCTOBER 2, 2024
The company, a provider of drug containment and delivery solutions, will introduce its expanded Vertiva 10mL on-body delivery system.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content