Bristol Myers CAR-T therapy approved by FDA for earlier myeloma use
Bio Pharma Dive
APRIL 5, 2024
The FDA’s clearance comes three weeks after a panel of advisers endorsed expanded use of Abecma despite safety concerns raised by the agency.

Bio Pharma Dive
APRIL 5, 2024
The FDA’s clearance comes three weeks after a panel of advisers endorsed expanded use of Abecma despite safety concerns raised by the agency.

Pharmaceutical Technology
APRIL 5, 2024
MiNA Therapeutics has entered into an agreement with Nippon Shinyaku to develop RNAa therapeutics for rare neurodegenerative ailments.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
APRIL 5, 2024
The German pharmaceutical company is “streamlining” its custom-facing teams behind Cyltezo, a copycat version of AbbVie’s immune disease drug Humira.

Pharma Times
APRIL 5, 2024
Hoxa9 and b-catenin molecules are a rare population of self-renewing HSCs found in bone marrow

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!
Pharmaceutical Technology
APRIL 5, 2024
Recent campaigns have targeted children and young adults in low vaccine uptake areas amidst a rising number of measles cases.

Pharma Times
APRIL 5, 2024
Affecting 80,000 people in England, 12% of brain tumour patients survive beyond five years of diagnosis
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Fierce Pharma
APRIL 5, 2024
Vertex CEO Reshma Kewalramani, M.D., made $20.6 million in 2023, which was an increase of 30% from her $15.9 million pay in 2022.

Worldwide Clinical Trials
APRIL 5, 2024
For many people, February 29th is simply Leap Day, an extraordinary date that appears only once every four years. For others, this rare day is aptly the day we honor rare disease communities. Observed annually on the last day of February since 2008, Rare Disease Day has grown into a global movement for raising awareness, promoting research, and advocating for improved access to rare disease treatments and support services.

Pharmaceutical Technology
APRIL 5, 2024
Despite a failed bid to expand Imfinzi’s treatment scope in NSCLC, the drug has proved effective in treating small cell lung cancer.

pharmaphorum
APRIL 5, 2024
Advances in immunosuppression research may bring transplant patients closer to “one organ for life” Mike.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
APRIL 5, 2024
GSK’s Trelegy Ellipta has been approved as a maintenance therapy for both asthma and COPD in Singapore.

Fierce Pharma
APRIL 5, 2024
After a short period of doubt, the FDA has followed the opinion of its advisers and moved Bristol Myers Squibb’s CAR-T therapy Abecma into the earlier treatment of multiple myeloma. | After a short period of doubt, the FDA has followed the opinion of its advisers and moved Bristol Myers’ CAR-T therapy Abecma into the earlier treatment of multiple myeloma.

Pharmaceutical Technology
APRIL 5, 2024
Last month, Amylyx announced that the Phase III confirmatory trial of ALS drug Relyvrio failed to meet its primary and secondary endpoints.

Bio Pharma Dive
APRIL 5, 2024
The deal comes just over a week after The Wall Street Journal reported the companies were discussing a merger.

Pharmaceutical Technology
APRIL 5, 2024
Acumen has entered a partnership with Lonza to further the development of sabirnetug, a potential treatment for Alzheimer's disease.

pharmaphorum
APRIL 5, 2024
ADRIATIC trial finds AstraZeneca's cancer immunotherapy Imfinzi improves survival in patients with limited-stage small cell lung cancer (SCLC)

Pharmaceutical Technology
APRIL 5, 2024
The UK MHRA has approved Advanz Pharma’s combined antibiotic, cefepime/enmetazobactam, to treat complicated infections.

pharmaphorum
APRIL 5, 2024
Germany's Merck partners Caris Discovery on artificial intelligence-powered discovery of antibody-drug conjugates in a $1.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
APRIL 5, 2024
Caris Life Sciences has entered a collaboration with Merck KGaA for the development of antibody-drug conjugates (ADCs) for cancer.

pharmaphorum
APRIL 5, 2024
Cancer Research UK has announced a £58.

Pharmaceutical Commerce
APRIL 5, 2024
In an interwith with Pharma Commerce Editor Nicholas Saraceno, Ed Schoonveld, Value and Access Advisor, Schoonveld Advisory, explains why pharmaceutical products tend to cost more in the US than the rest of the world.

pharmaphorum
APRIL 5, 2024
Our regular round-up of financings in the biotech sector is headed by rumours of a $150 to $200 million initial public offering (IPO) for Aardvark Therapeutics, an emerging player in the weight-loss category, alongside a trio of big private rounds.

Cloudbyz
APRIL 5, 2024
Implementing Electronic Data Capture (EDC) systems in clinical trials offers numerous benefits, including improved data accuracy, faster data availability, and streamlined processes. However, biotechnology sponsors face several practical challenges during its implementation. Here are ten of these challenges along with best practices to avoid or mitigate them: 1.

BioSpace
APRIL 5, 2024
The American Association for Cancer Research (AACR)’s annual conference runs April 5 through April 10. BioSpace will have all the key data and news out of San Diego.

Pharmaceutical Commerce
APRIL 5, 2024
The latest news for pharma industry insiders.

Fierce Pharma
APRIL 5, 2024
Inclusive Innovation: Key to the Future of Healthcare jpiatt Fri, 04/05/2024 - 10:37

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Drug Patent Watch
APRIL 5, 2024
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Netherlands.

Fierce Pharma
APRIL 5, 2024
AstraZeneca's Imfinzi has delivered positive clinical data in another type of lung cancer, building on the immunotherapy’s existing FDA clearance in stage 3 non-small cell lung cancer and extensive | AstraZeneca's Imfinzi has delivered positive clinical data in another type of lung cancer, building on the immunotherapy’s existing FDA clearance in stage 3 non-small cell lung cancer and extensive-stage small cell lung cancer.
Drug Channels
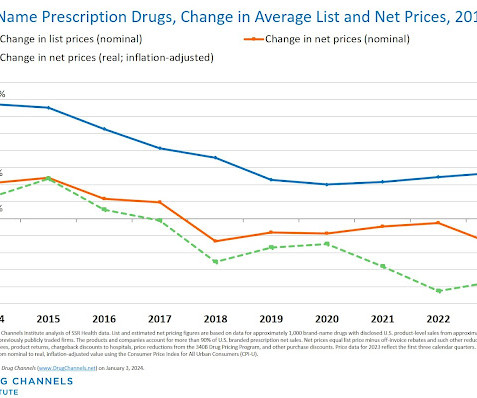
APRIL 5, 2024
This week, I’m rerunning some popular posts while I prepare for today’s live video webinar: Drug Channel Implications of the Inflation Reduction Act. One important update: In the article below, I suggested that the IRA will make high-list/high-rebate products less attractive to Medicare plans. But this appears not be accurate, as I explain in Surprise!

Fierce Pharma
APRIL 5, 2024
Amid ongoing drug shortages and other headline-grabbing issues that fall under the FDA’s purview, the House Committee on Oversight and Accountability is putting the agency’s commissioner Robert Cal | The April 11 hearing could be contentious, as the committee wants to “hold the commissioner accountable" for the FDA's action on drug shortages, among other issues, according to a statement from Chairman James Comer, R-Kentucky.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content