Vertex islet cell therapy gets type 1 diabetics off insulin
pharmaphorum
JUNE 23, 2024
Three people with type 1 diabetes who received Vertex Pharma’s islet cell therapy VX-880 were able to come off insulin within a year
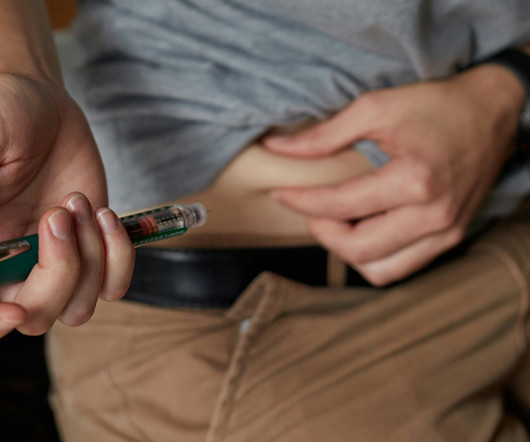
pharmaphorum
JUNE 23, 2024
Three people with type 1 diabetes who received Vertex Pharma’s islet cell therapy VX-880 were able to come off insulin within a year

JAMA Internal Medicine
JUNE 23, 2024
This case-control study estimates the association between receipt of the BNT162b2 XBB vaccine and medically attended COVID-19 outcomes among US adults 18 years and older.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
JUNE 23, 2024
Netherlands biotech Argenx has a second FDA approval for its FcRn inhibitor Vyvgart Hytrulo, adding a new indication in chronic inflammatory demyelinating polyneuropathy (CIDP) for a drug that it hopes could find a use in more than a dozen diseases.

JAMA Internal Medicine
JUNE 23, 2024
This qualitative study using a mixed-methods approach examines the identification of and proposed measures to address administrative harms.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

BioSpace
JUNE 23, 2024
5 FDA Decisions to Watch in the Second Half of 2024 6/24/2024
JAMA Internal Medicine
JUNE 23, 2024
This cohort study identifies the most potentially hepatotoxic medications based on clinical incidence rates of hospitalizations for severe acute liver injury (ALI) and examines how these rates compare with the hepatotoxicity categorization of severe ALI using published case reports.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Drug Patent Watch
JUNE 23, 2024
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Italy. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating for the long time needed to obtain regulatory approval for drugs.

BioSpace
JUNE 23, 2024
Lilly Sees Path for Zepbound’s Label Expansion With Phase III Sleep Apnea Win 6/24/2024

Drug Patent Watch
JUNE 23, 2024
Dabigatran etexilate mesylate is the generic ingredient in two branded drugs marketed by Alkem Labs Ltd, Apotex, Hetero Labs Ltd Iii, and Boehringer Ingelheim and, and is included in five NDAs. There are three patents protecting this compound. Drug patent litigation for DABIGATRAN ETEXILATE MESYLATE. Nine suppliers are listed for this compound.

BioSpace
JUNE 23, 2024
Altimmune Targets Obesity Market With Weight-Loss Drug That Preserves Lean Muscle Mass 6/24/2024

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Patent Watch
JUNE 23, 2024
MOVANTIK (naloxegol oxalate) Valinor Patent: 7,056,500 Expiration: Jun 29, 2024 See More … For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit www.DrugPatentWatch.

BioSpace
JUNE 23, 2024
G1 Therapeutics Fails Late-Stage Breast Cancer Trial, Plans ‘Targeted’ Headcount Reductions 6/24/2024

FDA Law Blog
JUNE 23, 2024
By John A. Gilbert & Karla L. Palmer — For more than 50 years, the Drug Enforcement Administration (DEA) has enforced the central mandate of the Controlled Substances Act (CSA) to maintain a closed chain of distribution for drugs with a potential for abuse and diversion. The CSA and regulations promulgated by DEA are intended to reduce the potential for diversion and abuse and ensure that controlled substances are dispensed and delivered to patients for a legitimate medical purpose.

BioSpace
JUNE 23, 2024
Novo’s Wegovy Shows Stronger Weight-Loss Effects in Women Than Men: Study 6/24/2024

BioSpace
JUNE 23, 2024
Alnylam Aces Phase III Cardio Trial, Eyes Label Expansion for RNAi Therapy 6/24/2024

BioSpace
JUNE 23, 2024
FDA Action Alert: Merck, Verona, AbbVie and Rocket 6/24/2024
Let's personalize your content