AstraZeneca sets sights on $80B in revenue by 2030
Bio Pharma Dive
MAY 21, 2024
The U.K. pharma expects to launch 20 new drugs by then, among them complex medicines for cancer as well as treatments for weight loss.

Bio Pharma Dive
MAY 21, 2024
The U.K. pharma expects to launch 20 new drugs by then, among them complex medicines for cancer as well as treatments for weight loss.

Pharmaceutical Technology
MAY 21, 2024
Roche announced an FDA breakthrough therapy designation for its inavolisib/Ibrance combination therapy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MAY 21, 2024
Since pivoting from oncology, the startup has raised close to $300 million and advanced an ulcerative colitis drug into mid-stage testing.
AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 21, 2024
The idea that the heart contains the very ‘essence’ of a person might be more than just a spiritual concept. Ever since the first human heart transplants back in 1967, patients have reported, often reluctantly, some eerie and inexplicable changes to their personalities.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
MAY 21, 2024
The U.K. drugmaker aims to catch up in developing new tumor-fighting technologies — areas where it has lagged behind leaders like Novartis and J&J.
Pharma Times
MAY 21, 2024
The funding aims to reduce health inequalities in these groups and save more lives
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
MAY 21, 2024
The network will support the development, evaluation and adoption of innovative health and care technology

Bio Pharma Dive
MAY 21, 2024
The collaboration, which gives Lilly access to Aktis’ technology in return for $60 million, adds to a flurry of dealmaking in the radiopharma field.

BioSpace
MAY 21, 2024
Patent cliffs and other factors may lead other large drugmakers to embrace similar cost-cutting measures, experts tell BioSpace.

Pharmaceutical Technology
MAY 21, 2024
Aktis will receive $60m in an upfront payment from Lilly, with the biotech eligible for up to $1.1bn in milestone payments and royalties.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

pharmaphorum
MAY 21, 2024
AstraZeneca CEO Pascal Soriot says group revenues can reach $80bn by 2030, fuelled by a pipeline that features 'many' potential $5bn-plus candidates.

Pharmaceutical Technology
MAY 21, 2024
The UK-based company plans to use the proceeds for financing the proof-of-concept clinical trials for its ADC pipeline.

pharmaphorum
MAY 21, 2024
FDA is asking artificial intelligence teams to develop a digital endpoint tool to study freezing of gait, a symptom of Parkinson’s that can have a serious impact on patients.

Pharmaceutical Technology
MAY 21, 2024
Larimar will continue the open label extension study in Friedreich’s ataxia with results expected in Q4 this year.

pharmaphorum
MAY 21, 2024
In today’s podcast, web editor Nicole Raleigh speaks with PASQAL’S technical business developer Europe, Krisztian Benyo, PhD, about the pharma applications and commercialisation of quantum science.

Pharmaceutical Technology
MAY 21, 2024
Glenmark has entered into an exclusive agreement with BeiGene to market and distribute two Beigene oncology medicines in India.

pharmaphorum
MAY 21, 2024
Despite the rate of bipolar disorder being the same among Black Americans as other Americans, there exists a significant disparity in diagnosis and subsequent treatment. Many Black Americans with bipolar disorder remain undiagnosed and untreated.

Pharmaceutical Technology
MAY 21, 2024
AmplifyBio and Xcell Biosciences have announced a strategic collaboration to manufacture engineered T-cell receptor (TCR) therapies.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
MAY 21, 2024
Going against the advice of the CHMP, the EU has said that PTC Therapeutics’ Translarna therapy for Duchenne muscular dystrophy should stay on the market

Pharmaceutical Technology
MAY 21, 2024
AstraZeneca is set to invest $1.5bn in a new manufacturing facility in Singapore dedicated to the production of antibody drug conjugates.

Drug Patent Watch
MAY 21, 2024
Annual Drug Patent Expirations for BELSOMRA Belsomra is a drug marketed by Merck Sharp Dohme and is included in one NDA. It is available from one supplier.
Pharmaceutical Technology
MAY 21, 2024
The US FDA has approved Yesafili and Opuviz, two interchangeable biosimilars to Eylea, for macular degeneration and other eye conditions.

Fierce Pharma
MAY 21, 2024
A decade ago, with AstraZeneca in decline and some investors urging the drugmaker to sell out to Pfizer, new CEO Pascal Soriot presented an audacious plan to hike revenue to $45 billion by 2023. | After AstraZeneca achieved Pascal Soriot's ambitious $45-billion-by-2023 revenue goal, the CEO is thinking big again. On Tuesday morning, AZ unveiled its new plan to swell its revenue to $80 billion by 2030.

Outsourcing Pharma
MAY 21, 2024
Clinical Trials Day is organized and promoted by the Association of Clinical Research Professionals (ACRP) for the last ten years.

BioSpace
MAY 21, 2024
Days after backing out of two Ionis-partnered neuro programs, Biogen has inked a potential $1.8 billion buy of Human Immunology Biosciences and boosting its late-stage immunology pipeline.

pharmaphorum
MAY 21, 2024
Digital investment in the pharma sector is continuing at a steady pace but could fall behind what is needed to deliver true transformation of their businesses.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
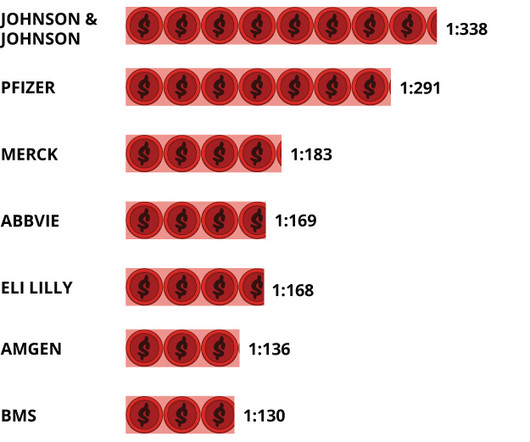
BioSpace
MAY 21, 2024
Despite weathering a difficult year, biopharma continues to see massive pay gaps between CEOs and their median employees, with top executives often earning hundreds of times more.

pharmaphorum
MAY 21, 2024
Discover how the Quillaja saponaria tree is revolutionising vaccine development as a sustainable, natural adjuvant. Learn about BSI's innovative work harnessing the power of this remarkable plant.

Rethinking Clinical Trials
MAY 21, 2024
May 19, 2024: The NIH Pragmatic Trials Collaboratory hosted a Pre-Conference Workshop at the 2022 Health Care Systems Research Network (HCSRN) Annual Conference. This training workshop introduces concepts in the design, conduct, and implementation of embedded pragmatic clinical trials (ePCTs), and provides firsthand ePCT experiences and case studies from the NIH Pragmatic Trials Collaboratory Trials.

Pharmaceutical Commerce
MAY 21, 2024
The latest news for pharma industry insiders.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content