Norgine seeks approval for high-risk neuroblastoma treatment
Pharmaceutical Technology
APRIL 16, 2024
Norgine has sought approval for eflornithine (DFMO) for the treatment of patients with high-risk neuroblastoma (HRNB).
Pharmaceutical Technology
APRIL 16, 2024
Norgine has sought approval for eflornithine (DFMO) for the treatment of patients with high-risk neuroblastoma (HRNB).

Bio Pharma Dive
APRIL 16, 2024
Joel Marcus, the longtime head of Alexandria Real Estate Equities, sees few near-term challengers to the established biotech hubs of Boston, San Diego and San Francisco.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
APRIL 16, 2024
The price ceiling policy has been in place for more than 20 years, but it has neither been very successful nor free of consequences for pharma companies.

Bio Pharma Dive
APRIL 16, 2024
Shares of the biotech rose 25% after a large trial found adding its drug Caplyta onto antidepressant therapy significantly helped people with major depression.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Pharmaceutical Technology
APRIL 16, 2024
NNC0519-0130 is commercialized by Novo Nordisk, with a leading Phase II program in Type 2 Diabetes.

Bio Pharma Dive
APRIL 16, 2024
The biotech says a dual-pronged antibody it’s developing could become an “off-the-shelf” alternative to cell therapies in development for lupus and other inflammatory conditions.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
APRIL 16, 2024
Solid organ transplantation is used to treat end-stage organ failure, including the kidneys

Pharmaceutical Technology
APRIL 16, 2024
Sanofi has signed an agreement with IDT Australia for preclinical formulation development of its messenger RNA (mRNA) vaccines.
Pharma Times
APRIL 16, 2024
Liver cirrhosis, caused by long-term liver damage, is estimated to affect around 100 million people worldwide

Bio Pharma Dive
APRIL 16, 2024
Tecvayli sales, which J&J broke out for the first time, surpassed Wall Street forecasts and offset underperformance from Carvykti, which J&J attributed to the "phasing and timing of orders.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

pharmaphorum
APRIL 16, 2024
A smart sensor developed by Adherium has been cleared by the FDA for use with AstraZeneca's asthma inhaler Airsupra and Breztri for COPD.

Pharmaceutical Technology
APRIL 16, 2024
Artificial intelligence (AI) has the potential to revolutionize drug development through improved efficiency, accuracy, and speed.

pharmaphorum
APRIL 16, 2024
Researchers from the UK have developed an endoscopic device that uses 3D imaging to look at the stiffness of cells and could diagnose cancer earlier.

Pharmaceutical Technology
APRIL 16, 2024
Acorda’s closure date is set for 15 June, as Merz Therapeutics looks set to acquire the biotech’s once-prolific assets.

pharmaphorum
APRIL 16, 2024
Boehringer Ingelheim says the strongest R&D pipeline in its history is set to deliver 25 new product launches between now and 2030

Pharmaceutical Technology
APRIL 16, 2024
Boehringer Ingelheim announced consistent growth across its human pharma sales and R&D investments in 2023.

Fierce Pharma
APRIL 16, 2024
Despite a pair of clinical misfires in 2023, argenx has stuck by the thesis that its star drug Vyvgart, also known as efgartigimod, could tackle a wide array of autoimmune diseases linked to immuno | Argenx is bolstering its case for Vyvgart’s CIDP potential and preparing for a possible launch later this year—all while the company continues to prove the medication’s worth in its inaugural generalized myasthenia gravis indication.

Pharmaceutical Technology
APRIL 16, 2024
The FDA has cleared Spinogenix’s Phase II trial of SPG601, as the European Commission grants orphan drug status to zatolmilast.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Drug Patent Watch
APRIL 16, 2024
The pharmaceutical industry in China is undergoing a significant transformation with the adoption of artificial intelligence (AI) technology.
Pharmaceutical Technology
APRIL 16, 2024
NovelMed has received ODD status from the US FDA for NM5072 for treating paroxysmal nocturnal hemoglobinuria (PNH).
pharmaphorum
APRIL 16, 2024
FDA starts review of GSK’s 5-in-1 meningitis vaccine, cueing up a decision early next year, as it tries to chase down Pfizer's recently-approved Penbraya

Pharmaceutical Technology
APRIL 16, 2024
The US FDA placed the clinical hold based on the preclinical data that showed that Neumora’s NMRA-266 triggered convulsions in rabbits.

Fierce Pharma
APRIL 16, 2024
Intra-Cellular Therapies has unveiled another positive late-stage trial readout for Caplyta, taking another step toward a potential blockbuster label expansion for the depression drug. | Intra-Cellular Therapies has unveiled another positive late-stage trial readout for Caplyta, taking another step toward a potential blockbuster label expansion for the depression drug.

Pharmaceutical Technology
APRIL 16, 2024
Qureight has secured $8.5m in a Series A funding round led by Hargreave Hale AIM VCT to accelerate drug development.

pharmaphorum
APRIL 16, 2024
Shares in Intra-Cellular Therapies rose after it reported Caplyta was effective for major depressive disorder (MDD), in a phase 3 trial.

Pharmaceutical Commerce
APRIL 16, 2024
The latest news for pharma industry insiders.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
APRIL 16, 2024
MK-1084 is a small molecule commercialized by Merck, with a leading Phase I program in Metastatic Colorectal Cancer.
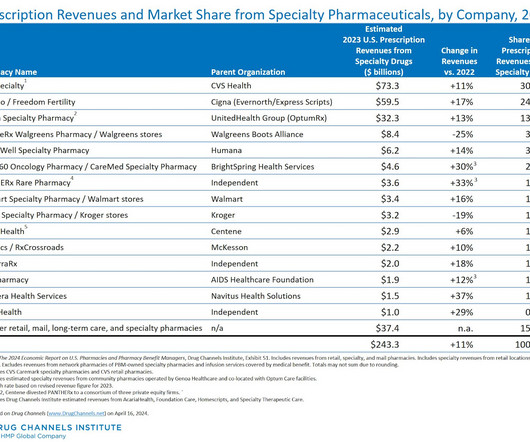
Drug Channels
APRIL 16, 2024
Drug Channels Institute’s (DCI’s) latest analysis finds that participants in the specialty pharmacy market continue to get more diverse—although revenues remain highly concentrated. We have identified nearly 1,800 dispensing sites with specialty pharmacy accreditation—about 40% of which are owned by hospitals, physician practices, and other healthcare providers.

Pharmaceutical Technology
APRIL 16, 2024
ABBV-400 is a monoclonal antibody conjugated commercialized by AbbVie, with a leading Phase II program in Metastatic Colorectal Cancer.

Outsourcing Pharma
APRIL 16, 2024
Brainbox Ltd is thrilled to announce its partnership with mBrainTrain, a collaboration poised to transform the landscape of neuroimaging technology.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content