Lilly builds case for weekly insulin shot
Bio Pharma Dive
SEPTEMBER 5, 2024
New data show Lilly’s longer-lasting insulin matched daily shots in controlling blood sugar, adding to positive findings the company disclosed in May.

Bio Pharma Dive
SEPTEMBER 5, 2024
New data show Lilly’s longer-lasting insulin matched daily shots in controlling blood sugar, adding to positive findings the company disclosed in May.

Pharmaceutical Technology
SEPTEMBER 5, 2024
Moderna is currently running a Phase I/II trial of the mRNA mpox vaccine mRNA-1769 in healthy participants.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
SEPTEMBER 5, 2024
The departure of Amy Emerson as CEO comes three weeks after the MDMA developer revealed plans to cut 75% of its staff.

Pharmaceutical Technology
SEPTEMBER 5, 2024
Eli Lilly and EVA Pharma have formed a partnership to increase the availability of baricitinib in 49 African countries by 2030.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!
Pharma Times
SEPTEMBER 5, 2024
Pre-clinical data of VTX-806 in demonstrated normalisation of metabolic parameters

Pharmaceutical Technology
SEPTEMBER 5, 2024
Haya could secure up to $1bn after hitting preclinical, clinical, and commercial milestones with drugs that emerge from this deal.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
SEPTEMBER 5, 2024
The UK government has taken a step towards banning xylazine and 21 other substances by introducing legislation in parliament.

Rethinking Clinical Trials
SEPTEMBER 5, 2024
In this Friday’s PCT Grand Rounds, Ardith Doorenbos of the University of Illinois, Chicago, and Diane Flynn of Madigan Army Medical Center will present “Conventional, Complementary, and Integrative Pain Therapies in a Military Population With Chronic Musculoskeletal Pain: Results of a Pragmatic Clinical Trial Using SMART Design.” The Grand Rounds session will be held on Friday, September 6, 2024, at 1:00 pm eastern.

pharmaphorum
SEPTEMBER 5, 2024
A "small number" of AstraZeneca employees have been detained in China in an investigation into suspected data privacy and drug import breaches
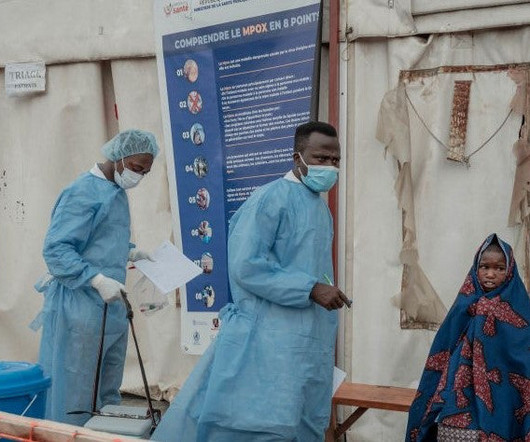
Pharmaceutical Technology
SEPTEMBER 5, 2024
Representatives of the WHO and the IFRC called for international cooperation to tackle the current mpox outbreak.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Fierce Pharma
SEPTEMBER 5, 2024
AstraZeneca has found its employees’ practices under the microscope of Chinese law enforcement years after an insurance fraud case. | AstraZeneca has found its employees’ practices under the microscope of Chinese law enforcement years after an insurance fraud case.

Pharmaceutical Technology
SEPTEMBER 5, 2024
A panel of researchers gave presentations on the clinical evidence for the role of RNA in therapy for cardiovascular conditions.

pharmaphorum
SEPTEMBER 5, 2024
Listen to the podcast interview with Manny Belabe from ArisGlobal as he discusses The Life Sciences Generative AI Council and its role in creating a sustainable technological future. Gain insights into the future of AI in life sciences.

Pharmaceutical Technology
SEPTEMBER 5, 2024
The subanalyses recontextualised data from the PACMAN-AMI trial on the therapeutic role of Praluent (alirocumab) in coronary heart disease.

pharmaphorum
SEPTEMBER 5, 2024
EU General Court suspends decision to revoke the license for Advanz Pharma's primary biliary cholangitis drug Ocaliva.

Pharmaceutical Technology
SEPTEMBER 5, 2024
eGenesis conducted the first-in-human porcine kidney transplant earlier this year, though the patient died two months later.

pharmaphorum
SEPTEMBER 5, 2024
ArsenalBio raises $325m for programmable CAR-T platform Phil.

Pharmaceutical Technology
SEPTEMBER 5, 2024
NHS England has announced the availability of MSD UK’s belzutifan, a take-at-home pill for patients with von Hippel-Lindau disease.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
SEPTEMBER 5, 2024
Medscape WebMD is a leading provider of medical information and services online, catering to the Medical Affairs field. Learn more about their company profile and offerings.

Pharmaceutical Technology
SEPTEMBER 5, 2024
The company has reduced its headcount by about 49% and suspended enrolment in a Phase II glioblastoma trial.

pharmaphorum
SEPTEMBER 5, 2024
Click Therapeutics' digital therapeutic for migraine clears late-stage study using an endpoint used to test drugs, clearing the way for regulatory filing.

Pharmaceutical Technology
SEPTEMBER 5, 2024
ArsenalBio has secured $325m in a Series C financing round to propel its programmable cell therapy programmes through clinical development.

pharmaphorum
SEPTEMBER 5, 2024
J&J is reported to have raised its offer to settle claims that its talc products caused cancer by $1.1bn, as it reaches agreement with a holdout law firm.

Fierce Pharma
SEPTEMBER 5, 2024
Johnson & Johnson’s newly FDA-approved lung cancer combination of Rybrevant and Lazcluze is inching ever closer to a statistically significant survival showing, which could help it better chall | Johnson & Johnson’s newly FDA-approved lung cancer combination of Rybrevant and Lazcluze is inching ever closer to a statistically significant survival showing, which could help it better challenge AstraZeneca’s Tagrisso.

Pharmaceutical Commerce
SEPTEMBER 5, 2024
In the second part of her interview with Pharma Commerce Editor Nicholas Saraceno, Ilisa B.G. Bernstein, President of Bernstein Rx Solutions, LLC, discusses the best practices that compliance teams should consider as the end of the stabilization period approaches this November.

Fierce Pharma
SEPTEMBER 5, 2024
With dwindling funds after a prior snub from the FDA, ImmunityBio’s journey to bag approval for its Merck-rivaling bladder cancer drug Anktiva wasn’t easy. | The drugmaker laid off 20 employees as it attempts to recoup from dwindling funds during its Anktiva launch in bladder cancer.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Commerce
SEPTEMBER 5, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.

Drug Patent Watch
SEPTEMBER 5, 2024
The pharmaceutical industry is a significant contributor to global carbon emissions, with a projected increase of over 300% by 2050 if left unchecked. This alarming trend has led to a growing need for sustainable practices in the industry, particularly in the development of generic drugs.

BioPharma Reporter
SEPTEMBER 5, 2024
The European Commission has granted Orphan Drug Designation for Vivet Therapeuticsâ gene therapy product VTX-806 for the treatment of Cerebrotendinous Xanthomatosis (CTX).

Fierce Pharma
SEPTEMBER 5, 2024
After handing over $30 million in criminal penalties last summer, Glenmark Pharmaceuticals has agreed to settle with the U.S. | Glenmark will pay the U.S. Department of Justice $25 million to resolve claims that it worked with other drugmakers to rig prices of the cholesterol med pravastatin. The case forms part of a larger antitrust probe implicating numerous manufacturers.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content