Galderma wins FDA approval for skin condition treatment
Bio Pharma Dive
AUGUST 13, 2024
Nemluvio is now cleared for adults with the chronic itching condition prurigo nodularis, making it a competitor to Sanofi and Regeneron’s Dupixent.

Bio Pharma Dive
AUGUST 13, 2024
Nemluvio is now cleared for adults with the chronic itching condition prurigo nodularis, making it a competitor to Sanofi and Regeneron’s Dupixent.

Pharmaceutical Technology
AUGUST 13, 2024
GLP-1RAs may be more effective for patients with immune diseases and type 2 diabetes than DPP-4 inhibitors, as per analysed health records.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
AUGUST 13, 2024
The company will no longer develop its medicine izokibep in two immune conditions, prioritizing instead another treatment it’s developing for thyroid eye disease.
Pharmaceutical Technology
AUGUST 13, 2024
Ascendis Pharma has obtained US Food and Drug Administration (FDA) approval for its YORVIPATH to treat hypoparathyroidism in adults.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
AUGUST 13, 2024
Berta Rodriguez-Hervas has joined the pharmaceutical company after stints at Stellantis, Nvidia and Tesla.

Pharma Times
AUGUST 13, 2024
The rare, peripheral neuropathy disease affects nearly three million people globally
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharma Times
AUGUST 13, 2024
The V-ATPase protein, V1H, was found to be involved in the process of raising the alarm

Pharmaceutical Technology
AUGUST 13, 2024
Baxter is handing its kidney care department over to investment firm Carlyle, the company will carry on as its own entity known as Vantive.

pharmaphorum
AUGUST 13, 2024
With the implementation of advancing technologies in industry comes the parallel need for suitable upskilling of the workforce – but this doesn’t as yet always pan out, and in today’s pharmaphorum podcast COO of Enthought Mike Connell discusses the growing skills gap in R&D in life sciences and the 80/20 rule with web editor Nicole Raleigh, as well as the need for correct education of people and machines, both.
Pharmaceutical Technology
AUGUST 13, 2024
In March 2024, Madrigal Pharmaceuticals had a breakthrough moment when, after years of research and anticipation, the US Food and Drug Administration (FDA) granted approval for its Rezdiffra (resmetirom), a pioneering treatment for adults with F2-F3 metabolic dysfunction-associated steatohepatitis (MASH).

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Fierce Pharma
AUGUST 13, 2024
When the FDA approved Amgen’s Imdelltra in May, the T-cell engager was hailed as a breakthrough for the treatment of small cell lung cancer (SCLC) and as the first DLL3-targeting therapy of potenti | In granting an accelerated approval to Amgen's first-in-class DLL3 bispecific Imdelltra, the FDA had to work through a “large number” of missing adverse events from a pivotal trial.

Pharmaceutical Technology
AUGUST 13, 2024
The court rebuffed claims by Novartis that it would experience “irreparable harm” in the absence of an injunction following MSN’s generic roll-out.
BioPharma Reporter
AUGUST 13, 2024
The FDA has given the green light to Lymphir, the first immunotherapy for the treatment of cutaneous T-cell lymphoma (CTCL) to get approval in over five years.

Pharmaceutical Technology
AUGUST 13, 2024
Astria Therapeutics has selected Ypsomed’s Ypsomate autoinjector as its partner for the administration of STAR-0215 for the treatment of hereditary angioedema (HAE).
pharmaphorum
AUGUST 13, 2024
Learn how real-world evidence platforms use real-world data (RWD) to advance precision medicine, bridging the gap between clinical trials and real-world patient outcomes.

Pharmaceutical Technology
AUGUST 13, 2024
The incorporation of cognition into the safety profiles of both CNS and non-CNS indications is becoming increasingly critical.

pharmaphorum
AUGUST 13, 2024
Our longtime Finance Director Paul Bannister was a part of the fabric of pharmaphorum almost from the beginning and an integral part of keeping the publication running. And, most importantly, he was the soul of the place and a friend to all.

Pharmaceutical Technology
AUGUST 13, 2024
Syros discontinued enrolment in an acute myeloid leukaemia (AML) trial after a futility analysis found the study was unlikely to succeed.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Commerce
AUGUST 13, 2024
The latest news for pharma industry insiders.
Pharmaceutical Technology
AUGUST 13, 2024
The US FDA has approved Galderma’s Nemluvio as a pre-filled pen for subcutaneous injection to treat adult people with prurigo nodularis.

BioPharma Reporter
AUGUST 13, 2024
Driven by personal loss and inspired by the progress in disease management, Elaine has dedicated more than 20 years to the clinical trial technology industry.

Pharmaceutical Technology
AUGUST 13, 2024
The China NMPA has accepted for review Junshi Biosciences’ sNDA for toripalimab to treat unresectable or metastatic melanoma.

pharmaphorum
AUGUST 13, 2024
Novartis' attempt to stop MSN Pharma from launching a generic version of heart failure blockbuster Entresto is blocked in a US court
Pharmaceutical Technology
AUGUST 13, 2024
Citius Pharmaceuticals has concluded the merger of its oncology subsidiary with TenX Keane Acquisition, forming Citius Oncology.

Outsourcing Pharma
AUGUST 13, 2024
In the rapidly evolving pharmaceutical industry, the process of evaluating outsourcing partners is critical to ensuring quality, efficiency, and innovation.
BioPharma Reporter
AUGUST 13, 2024
The US approval of Nemluvio marks a major milestone for Galderma, a Swiss company developing treatments for a wide range of conditions affecting the skin.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
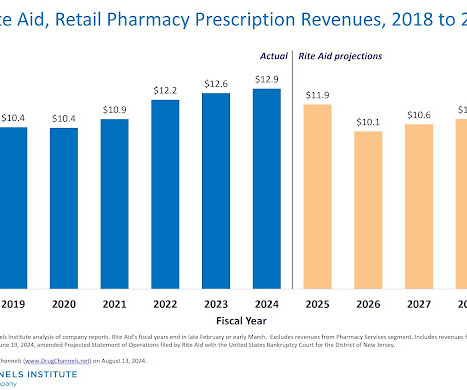
Drug Channels
AUGUST 13, 2024
Last fall, poor ol’ Rite Aid finally succumbed to bankruptcy. It was pretty much the definition of an expected surprise. To get a comprehensive look at the company’s ever-declining fortunes, DCI rummaged around the compnay's numerous bankruptcy filings. Below, you’ll find our review of Rite Aid’s current financial situation, shrinking store footprint, changing relationship with key wholesaler McKesson, surprisingly optimistic projections, and more.
BioPharma Reporter
AUGUST 13, 2024
Angiex has announced the beginning of the first-in-human trial of AGX101, an antibody-drug conjugate (ADC) treatment that targets solid tumors with a unique mode of action.
Outsourcing Pharma
AUGUST 13, 2024
Patients suffering from severe allergic reactions now have access to a new, needle-free treatment option, as the US Food and Drug Administration (FDA) has approved neffy, a 2 mg epinephrine nasal spray developed by ARS Pharmaceuticals, Inc.

Fierce Pharma
AUGUST 13, 2024
Despite winning an FDA approval in 2023 and gaining clarity in May on the regulatory path forward for its Type 1 diabetes prospect sotagliflozin, Lexicon Pharmaceuticals hasn’t had an easy go of it | As part of a restructuring initiative, Lexicon Pharmaceuticals will lay off more than 75 staffers, or approximately 50% of its current field force, by the end of September.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content