Into the unknown: How quickly can vaccines be developed for Disease X
Pharmaceutical Technology
SEPTEMBER 13, 2024
R&D frameworks are in place for the next pandemic-causing pathogen, but ways to derisk development are unclear.

Pharmaceutical Technology
SEPTEMBER 13, 2024
R&D frameworks are in place for the next pandemic-causing pathogen, but ways to derisk development are unclear.
AuroBlog - Aurous Healthcare Clinical Trials blog
SEPTEMBER 13, 2024
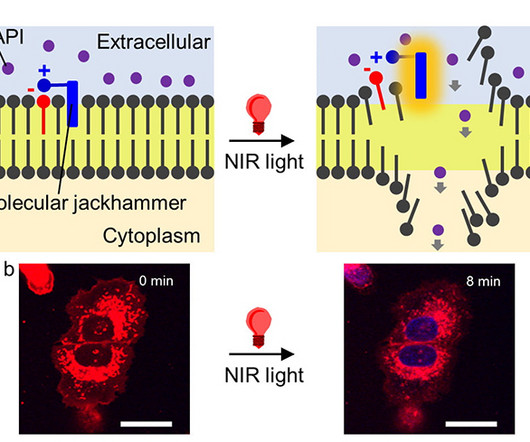
Illustration of a cancer cell. (Science Photo Library/Canva Pro) Scientists have discovered a remarkable way to destroy cancer cells. Stimulating aminocyanine molecules with near-infrared light caused them to vibrate in sync, enough to break apart the membranes of cancer cells. [link] Aminocyanine molecules are already used in bioimaging as synthetic dyes.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
SEPTEMBER 13, 2024
Distribution of the self-amplifying mRNA vaccine Kostaive is set to align with a planned October 2024 Covid-19 vaccination campaign.

Bio Pharma Dive
SEPTEMBER 13, 2024
During an investor presentation Thursday, executives admitted to being overly optimistic their vaccine could wrest significant market share away from GSK's and Pfizer's products this year.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Pharmaceutical Technology
SEPTEMBER 13, 2024
The US Food and Drug Administration (FDA) has approved Roche's Tecentriq Hybreza, a subcutaneous anti-PD-(L)1 cancer immunotherapy.

Bio Pharma Dive
SEPTEMBER 13, 2024
Roche has now beat Merck and Bristol Myers to market with its under-the-skin immunotherapy. Elsewhere, Sanofi bought into radiopharmaceuticals and Mene Pangalos joined Biogen's board.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
SEPTEMBER 13, 2024
Oncternal is halting the development of two cancer candidates, ONCT-534 and ONCT-808, after disappointing trial results.

Pharma Times
SEPTEMBER 13, 2024
Innovative immunotherapy drug HTL0039732 shows potential in trials

Pharmaceutical Technology
SEPTEMBER 13, 2024
The company plans to focus its resources on conducting clinical trials for CD388 influenza prevention therapy.

Fierce Pharma
SEPTEMBER 13, 2024
It’s better late than never for an FDA approval for the first subcutaneous PD-L1 inhibitor, which was doled out to Roche’s Tecentriq Hybreza after manufacturing delays derailed the company's initia | The under-the-skin formulation trims administration time down to seven minutes compared to the 30 to 60 minutes needed for intravenous Tecentriq.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
SEPTEMBER 13, 2024
Lilly has announced a significant expansion of its manufacturing presence in Ireland, with a $1bn investment earmarked for its Limerick site.

pharmaphorum
SEPTEMBER 13, 2024
AI-driven solutions are revolutionising the pharmaceutical supply chain management process, particularly in reducing drug shortages. Learn more about the power of AI in this field.

Pharmaceutical Technology
SEPTEMBER 13, 2024
Sanofi has signed a licensing agreement with RadioMedix and Orano Med to advance a radioligand therapy, AlphaMedix.

Fierce Pharma
SEPTEMBER 13, 2024
In a rough day for PARP inhibitors in ovarian cancer, GSK’s Zejula didn’t deliver a life extension benefit in the final analysis of a phase 3 trial. | In a rough day for PARP inhibitors in ovarian cancer, GSK’s Zejula didn’t deliver a life extension benefit in the final analysis of a phase 3 trial. Another study showed that Pharma&’s attempt to enhance its Rubraca with Bristol Myers Squibb’s Opdivo seriously backfired.

Pharmaceutical Technology
SEPTEMBER 13, 2024
Sobi and Enable Injections have entered an agreement for the development and distribution of Aspaveli in combination with enFuse.

pharmaphorum
SEPTEMBER 13, 2024
It's known that excessive use of earbuds at high volumes can cause hearing loss, so somewhat ironic that the FDA has just authorised software that can turn Apple AirPods into hearing aids.The green light is for Hearing Aid Feature (HAF), an app compatible with Apple's AirPods Pro earbuds that amplifies sounds and can be used for people aged 18 and over with perceived mild to moderate hearing impairment.
Fierce Pharma
SEPTEMBER 13, 2024
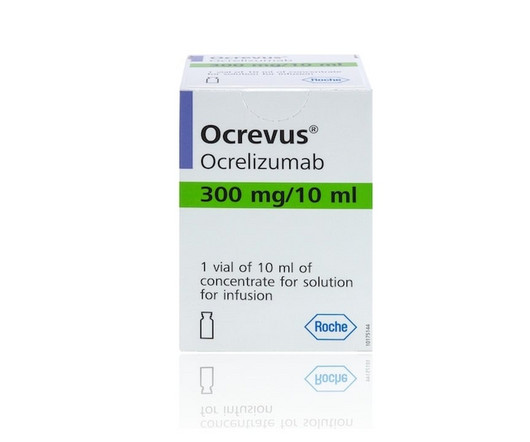
When Roche's Genentech gained approval for Ocrevus in 2017, the first-in- | The day after Roche gained an FDA approval for its subcutaneous version of cancer drug Tecentriq, the company also scored with a U.S. nod for its under-the-skin formulation of mega-blockbuster multiple sclerosis drug Ocrevus. The approval gives patients a more convenient injected way to receive Ocrevus as opposed to the infused formulation.

pharmaphorum
SEPTEMBER 13, 2024
Eli Lilly has added $1 billion to its investment plan for a new manufacturing facility in Ireland that will be used to produce its Alzheimer's therapy donanemab.The facility at a business park in Raheen, Limerick, was first announced in 2022 and this is the second time that Lilly has opted to double its investment in the site, taking the total above $2 billion and reinforcing the company's close ties to Ireland's pharma manufacturing sector.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Fierce Pharma
SEPTEMBER 13, 2024
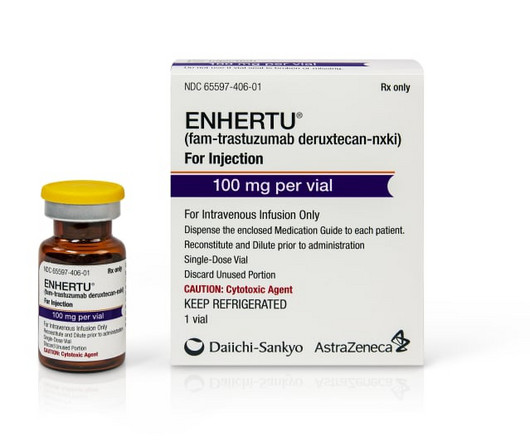
Daiichi Sankyo and AstraZeneca are padding the case for their antibody-drug conjugate Enhertu with new data demonstrating the medicine’s worth in patients whose cancer has spread to the b | To bolster Enhertu's case in HER2-positive breast cancer, AstraZeneca and Daiichi Sankyo have rolled out positive data for the antibody-drug conjugate in patients who have brain metastases.

XTalks
SEPTEMBER 13, 2024
In 2024, the healthcare industry is not just about innovation; it’s about making a real-world impact. From cutting-edge digital health solutions to tackling health disparities head-on, the largest healthcare companies are reshaping how care is delivered. This blog explores the top 10 largest publicly traded healthcare companies by market capitalization , making strides in healthcare delivery and innovation. 1.

pharmaphorum
SEPTEMBER 13, 2024
The PM Society Digital Awards 2024, an annual event in the healthcare marketing industry sponsored by Twist Health, held its 15th ceremony at The Brewery in London on 12th September.

Fierce Pharma
SEPTEMBER 13, 2024
ESMO: Novartis, Daiichi, Gilead, Astellas and AZ excel in analysis of clinical value added from new cancer approvals kdunleavy Fri, 09/13/2024 - 07:38

pharmaphorum
SEPTEMBER 13, 2024
Cellino Biotech has received funding from the US government to develop a scalable biomanufacturing platform for personalised regenerative medicines derived from patients' own stem cells.The $25 million award from the Advanced Research Projects Agency for Health (ARPA-H) will be used to develop Cellino's NEBULA system, which according to the firm's chief technology Matthias Wagner can "effectively print new cells and new tissues for patients using their own DNA.

Fierce Pharma
SEPTEMBER 13, 2024
Even for Merck’s oncology powerhouse Keytruda, the emerging role of immunotherapies in gynecological cancer treatment represents a bit of a mixed bag. | The company unveiled new data from its KEYNOTE-A18 study in late stage cervical cancer and its failed KEYNOTE-B21 trial testing Keytruda as an adjuvant therapy in newly diagnosed endometrial cancer.

Drug Channels
SEPTEMBER 13, 2024
Today’s guest post comes from Rick Fry, VP of Strategic Development at GoodRx. Rick explains why patients and pharmaceutical manufacturers now pay a greater share of prescription costs. He outlines GoodRx's services that address the twin challenges when the patient is the payer and the manufacturer faces intense gross-to-net pressures. To learn more, download the GoodRx case study: GoodRx Integrated Platform Solutions: Patient Navigator + Integrated Copay Card.

Fierce Pharma
SEPTEMBER 13, 2024
As if a limited initial FDA approval was not bad enough, AstraZeneca’s Truqap has recorded a pivotal trial flop that could raise additional doubts around the first-in-class AKT inhibitor. | AstraZeneca is detailing Truqap's pivotal trial flop in triple-negative breast cancer, a result that could raise additional doubts around the first-in-class AKT inhibitor.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
pharmaphorum
SEPTEMBER 13, 2024
UK biotech F2G has raised $100 million as it prepares to refile its olorofim candidate for invasive fungal infections, which was turned down last year by the FDA.The Manchester-based company said the new cash will help it to complete the late-stage development of olorofim, a novel oral antifungal for invasive aspergillosis and other serious fungal infections, to support the company during the regulatory review process, and prepare for a commercial launch in the US.

Sciensano
SEPTEMBER 13, 2024
Recent research provides critical insights into the impact of air pollution and high ambient temperatures on the health of Belgian citizens , emphasizing the need for long-lasting and effective measures to reduce air pollution in Belgium. In Belgium, air pollution from road traffic is a major environmental health risk. It consists of pollutants such as nitrogen dioxide, fine particulate matter, and benzene, all of which are known to cause serious health issues, including respiratory and cardiova

XTalks
SEPTEMBER 13, 2024
Diverse clinical trials are a matter of equity and essential for the validity and reliability of research outcomes. Historically, racial and ethnically minoritized groups or populations have been excluded from clinical trials, leading to a lack of data on how different demographic subgroups respond to treatments. The Clinical Trials Transformation Initiative (CTTI) aims to change this narrative by developing recommendations and driving the adoption of practices that support inclusivity and repre
WCG Clinical
SEPTEMBER 13, 2024
Traumatic brain injury (TBI) is a devastating clinical condition that contributes to a substantial number of deaths and cases of permanent disability. Each year over 1.5 million individuals in the United States and approximately 10 million people worldwide suffer a TBI and it has now has become one of the leading causes of morbidity and mortality in children and young adults.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content