Delayed CDC meeting on vaccines is rescheduled to April
Bio Pharma Dive
MARCH 21, 2025
A panel of CDC advisers, who had been set to meet in February, will discuss the current measles outbreak as well as guidelines for several types of shots.

Bio Pharma Dive
MARCH 21, 2025
A panel of CDC advisers, who had been set to meet in February, will discuss the current measles outbreak as well as guidelines for several types of shots.

Pharmaceutical Technology
MARCH 21, 2025
J&J is the latest pharma company to announce a major US investment, committing to a $55bn influx in the next four years.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Channels
MARCH 21, 2025
Todays guest post comes from Greg Skalicky, President of EVERSANA. Greg discusses some of the challenges manufacturers face with product commercialization, patient access and adherence, and negotiating partnerships with pharmacy benefit managers (PBMs). He introduces us to EVERSANA DIRECT Commercialization, a direct-to-patient change/model. To learn more about EVERSANA DIRECT Commercialization and how the direct-to-patient model can help you, meet with EVERSANA at the Asembia Summit in Las Vegas
Pharmaceutical Technology
MARCH 21, 2025
Menarini Group has announced a partnership with VisualDx to enhance the diagnosis of individuals with BPDCN.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
MARCH 21, 2025
Initially, Amvuttra’s annual list price will be nearly double the yearly cost of rival medicines from Pfizer and BridgeBio. But executives argue its “compelling and highly differentiated value” justify the higher charge.

Pharmaceutical Technology
MARCH 21, 2025
J&J has received US FDA approval for Tremfya (guselkumab), to treat moderately to severely active Crohn's disease (CD) in adults.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
MARCH 21, 2025
Ahead of World Tuberculosis Day on 24 March, the WHO has said that global funding cuts are curtailing efforts to fight the disease.

Bio Pharma Dive
MARCH 21, 2025
The pharma is spending $2.5 billion on an R&D center in Beijing. Elsewhere, Adaptimmune warned it could run out of cash and Pfizer sold its remaining stake in Haleon.

Pharmaceutical Technology
MARCH 21, 2025
Understanding reuse rights and copyright compliance is critical, particularly in todays ever-evolving technological landscape.

Bio Pharma Dive
MARCH 21, 2025
Proposed cuts to National Institutes of Health funding threaten innovation necessary to fuel advances in patient care, researchers and provider groups say.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Pharmaceutical Technology
MARCH 21, 2025
Shorter trial authorisation timelines for ATMP trials in the US are an advantage for biotechs, say experts.

Bio Pharma Dive
MARCH 21, 2025
The PBM is pivoting to a cost-based reimbursement model, which it says will reduce variation in how pharmacies are paid. Pharmacy groups say that depends on the specifics of Optum Rx’s plan.

Pharmaceutical Technology
MARCH 21, 2025
Clinical trials must use effective electronic data capture platforms that prioritise data integrity and comply with regulations.

XTalks
MARCH 21, 2025
Each year, Fast Company celebrates creativity and problem-solving with its annual Worlds Most Innovative Companies list, spotlighting those shaping the future across various industries. Here we spotlight the top 20 most innovative pharma and biotech companies of 2025 from their 2025 list that are transforming health care. The rankings are based on each companys ability to tackle critical challenges from drug delivery and diagnostics to personalized therapies using cutting-edge science.
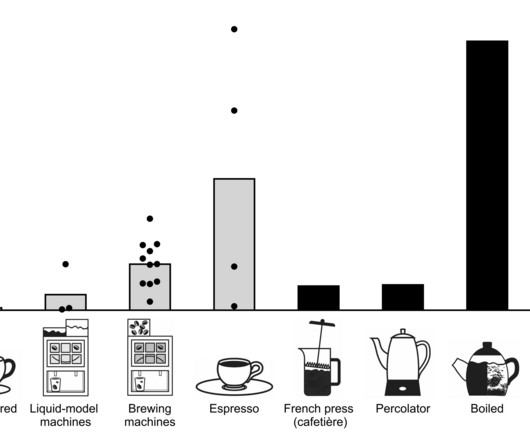
Medical Xpress
MARCH 21, 2025
The coffee from most of the coffee machines in workplaces contains relatively high levels of cholesterol-elevating substances. There is a big difference in comparison to coffee made in regular paper filter coffee makers, which filter out most of these substances.

pharmaphorum
MARCH 21, 2025
Alnylam has claimed FDA approval for Amvuttra in ATTR cardiomyopathy, allowing it to compete with rival therapies from Pfizer and BridgeBio.

Medical Xpress
MARCH 21, 2025
Imagine a boxer dodging a punch, a musician perfectly timing a note, or a driver anticipating a green lightthe brain can be seen as an amazing tool that is constantly predicting the future. But how does it do this?
pharmaphorum
MARCH 21, 2025
Novartis' oral complement inhibitor Fabhalta has become the first approved treatment for rare kidney disease C3G as it chases a $3bn sales target

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Medical Xpress
MARCH 21, 2025
A research team led by Mount Sinai has uncovered mechanisms of abnormal immune cell function that may lead to Crohn's disease, according to findings published in Science Immunology. The researchers said their discovery provides a better understanding of disease development and could inform the development and design of new therapies to prevent inflammation before it starts in the chronic disorder.

pharmaphorum
MARCH 21, 2025
Pharma is running out of names. Can an AI help? Jonah.

Medical Xpress
MARCH 21, 2025
New research to be presented at this year's European Congress on Obesity (ECO 2025, Malaga, Spain, 1114 May) shows that having an overweight or obese trajectory during childhood is associated with an increased risk of chronic obstructive pulmonary disease (COPD) in adulthood.

pharmaphorum
MARCH 21, 2025
AstraZeneca will open its sixth R&D centre in China, while J&J announces four new manufacturing facilities in the US in a major reshoring drive.

Medical Xpress
MARCH 21, 2025
A new study by UCLA Health has discovered what researchers say is the first drug to fully reproduce the effects of physical stroke rehabilitation in model mice.
pharmaphorum
MARCH 21, 2025
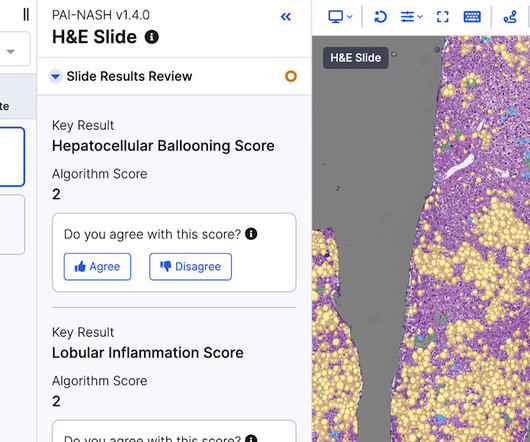
PathAI's artificial intelligence tool for diagnosing MASH has become the first to be recommended under the EMA's Qualification Opinion framework.

Medical Xpress
MARCH 21, 2025
In a study on ovarian cancer cells, researchers from Karolinska Institutet demonstrate how the tumor environment influences how cancer cells respond to drugs by using AI. The study has been published in the journal Communications Biology.
pharmaphorum
MARCH 21, 2025
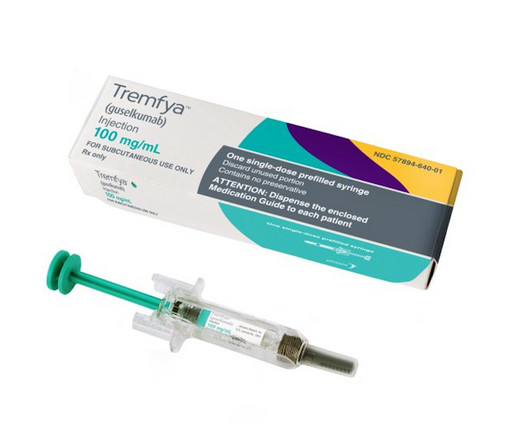
J&J's Tremfya gets a much-anticipated FDA approval for Crohn's disease, which the company thinks will be key to a renewed phase of sales growth.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Medical Xpress
MARCH 21, 2025
Patients hoping for a kidney transplant must first undergo a battery of medical tests to determine whether they are suitable candidates for the procedure and healthy enough to take post-transplant immunosuppressant drugs to prevent organ rejection.

Medical Xpress
MARCH 21, 2025
A study in the journal Diabetes Technology & Therapeutics (DTT) concludes that inhaled technosphere insulin (TI-Afrezza) should be considered as an option for individuals with type 1 diabetes who want an alternative to using an insulin pump or multiple daily injections (MDI) for insulin delivery.

Pharmaceutical Commerce
MARCH 21, 2025
Jonathan James, CEO, Hope Charities, discusses the challenge of nonprofits securing sustainable funding while ensuring patients are aware of available assistance.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content