AbbVie and Umoja to develop new CAR-T therapies for cancer
Pharmaceutical Technology
JANUARY 5, 2024
AbbVie and Umoja Biopharma partnered for the development of in-situ CAR-T cell therapy candidates for oncology targets.

Pharmaceutical Technology
JANUARY 5, 2024
AbbVie and Umoja Biopharma partnered for the development of in-situ CAR-T cell therapy candidates for oncology targets.

Bio Pharma Dive
JANUARY 5, 2024
Lykos Therapeutics, formerly known as MAPS Public Benefit Corp., recently submitted its MDMA capsules for FDA approval, following two positive studies.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JANUARY 5, 2024
Intellia Therapeutics announced its clinical goals for the next few years, pushing development for its late-stage ATTR asset.

Fierce Pharma
JANUARY 5, 2024
Three days after the FDA included Novo Nordisk’s semaglutide treatments Ozempic and Wegovy on a list of medicines that it would monitor for side effects—one of them suicide ideation—a study of medi | Three days after the FDA included Novo Nordisk’s semaglutide treatments Ozempic and Wegovy on a list of medicines that it would monitor for side effects—one of them suicide ideation—a study of medical records of patients shows no link between use of the GLP-1 drugs and an increase in suicidal though

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JANUARY 5, 2024
A BBC team was able to buy over 1600 prescription pills by supplying fake data, raising concerns over limited oversight.

Pharma Mirror
JANUARY 5, 2024
New Delhi, India – FD-AID, LLC, a distinguished leader in pharmaceutical quality and compliance solutions, proudly announces its entry into the Indian pharmaceutical market. Spearheaded by a former US FDA Investigator and seasoned pharmaceutical executive, FD-AID is uniquely positioned to bring a wealth of expertise and insight to India’s rapidly growing pharmaceutical industry.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Antidote
JANUARY 5, 2024
Though clinical trial patient recruitment is an integral part of the medical research process, it is often one of the most difficult aspects of conducting a study. There are various reasons for this challenge, and among them is the widespread existence of misconceptions about participating in research that many patients may see as a barrier to entry.

Pharmaceutical Technology
JANUARY 5, 2024
Menarini subsidiary Stemline will receive upfront and milestone payments, potentially worth more than $500m, along with royalties from sales.

pharmaphorum
JANUARY 5, 2024
Explore the highlights of the 2023 Benchling biotech press roundtable before Benchtalk London, showcasing cutting-edge software and innovations in the field of biotechnology.

Pharmaceutical Technology
JANUARY 5, 2024
Rhythm to acquire global rights for South Korean LG Chem’s oral obesity drug LB54640 for $100m in cash and equity.

Pharma Times
JANUARY 5, 2024
Vd1-gd T cells were effective in predicting positive responses to ICI therapy

Pharmaceutical Technology
JANUARY 5, 2024
KAI, a technology company based in the UK, is a Category Award Winner for Innovation in the 2023 Pharmaceutical Technology Excellence Awards

Pharma Times
JANUARY 5, 2024
The companies will aim to select targets, discover and develop new therapeutics

Pharmaceutical Technology
JANUARY 5, 2024
MOMA Therapeutics and Roche have signed a strategic partnership and licensing agreement for discovering drug targets for cancer.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
JANUARY 5, 2024
Gain insights from sustainability leaders in the life sciences industry on navigating the path to sustainable pharma. Learn about their efforts in addressing environmental issues, social impact, governance practices, and enhancing patient outcomes.

Pharmaceutical Technology
JANUARY 5, 2024
Avistone has closed its Series B financing round, raising 1bn yuan ($140m), to support clinical research and development of its drug pipeline.

Pharmaceutical Commerce
JANUARY 5, 2024
LillyDirect will facilitate access to medications by connecting patients with independent telehealth providers, bypassing the need to obtain a prescription from a physician and then going to a pharmacy to fill it.

Pharmaceutical Technology
JANUARY 5, 2024
Boehringer Ingelheim has signed a deal with 3T Biosciences for the discovery and development of next-generation cancer immunotherapies.

Fierce Pharma
JANUARY 5, 2024
When Theravance called it quits on a phase 2 JAK inhibitor program last February, the South San Francisco and Dublin-based biopharma company put the majority of its chips on a head-to-head trial pi | In the phase 4 study, Theravance and Viatris’ Yupelri failed to demonstrate greater lung function improvement in adults with COPD versus Spiriva delivered by a dry powder inhaler, Theravance said Friday.

pharmaphorum
JANUARY 5, 2024
Beckley Psytech has announced that is has received substantial strategic investment from atai Life Sciences, totalling $50 million, to accelerate the clinical development of short-duration psychedelics.

Fierce Pharma
JANUARY 5, 2024
The last few days of 2023 and the beginning of 2024 featured a flurry of dealmaking by the likes of AstraZeneca, Johnson & Johnson, Roche and Boehringer Ingelheim with Asian companies | A flurry of deals were inked by the likes of AstraZeneca, Johnson & Johnson, Roche and Boehringer Ingelheim with Asian companies. Big Pharma companies are also increasingly entrusting commercialization of some products in China to domestic firms.

XTalks
JANUARY 5, 2024
OAKBERRY, a leading Brazilian global açaí brand, has successfully raised $67 million in Series C funding. This significant financial milestone was led by the Brazilian investment bank, BTG Pactual. The capital raised will be set aside to accelerate OAKBERRY’s global expansion strategy, with a keen focus on the US market. The funds are strategically intended to bolster OAKBERRY’s revenue to an impressive $200 million.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
JANUARY 5, 2024
UCB is heading into 2024 with several new approvals under its belt, but it’s leaving its membership in the Biotechnology Innovation Organization (BIO) behind. | Both companies opted not to renew their 2024 memberships with the Biotechnology Innovation Organization (BIO). UCB will still retain its membership in other top trade groups, including the Pharmaceutical Research and Manufacturers Association of America (PhRMA).

Drug Patent Watch
JANUARY 5, 2024
This chart shows the drugs with the most patents in Cyprus. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.… The post Which pharmaceutical drugs have the most drug patents in Cyprus? appeared first on DrugPatentWatch - Make Better Decisions.

XTalks
JANUARY 5, 2024
Eli Lilly launched LillyDirect this week, an online service that provides access to the company’s medicines, including its newly approved weight loss drug Zepbound (tirzepatide). The website, according to the announcement from Lilly , offers an end-to-end digital healthcare experience and will provide patients access to its diabetes, weight loss and migraine medications, among others.

Drug Patent Watch
JANUARY 5, 2024
Annual Drug Patent Expirations for BRYHALI Bryhali is a drug marketed by Bausch and is included in one NDA. It is available from one supplier. There are two patents protecting… The post New patent for Bausch drug BRYHALI appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Commerce
JANUARY 5, 2024
Trials will evaluate four novel agents for cancers that include essential thrombocythemia, chronic lymphocytic leukemia, small lymphocytic lymphoma, non-small cell lung cancer, endometrial carcinoma, and metastatic castration-resistant prostate cancer.

pharmaphorum
JANUARY 5, 2024
Experienced executive Sarah Alwardt brings data and technology to the forefront in her dual role as President of Avalere and U.S.

Pharmaceutical Commerce
JANUARY 5, 2024
In the midst of being approved for commercial manufacturing of Pluvict, the 70,000 square-foot site was built specifically to accommodate RLT production.
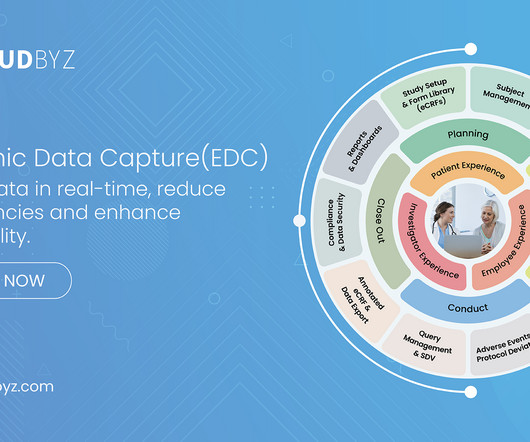
Cloudbyz
JANUARY 5, 2024
Cloudbyz EDC is a user-friendly, cloud-based solution that is designed to capture and manage clinical data effectively throughout a clinical trial’s life cycle. Our innovative solution enables clinical research teams to efficiently collect, analyze, and manage clinical data of different complexity and size. Cloudbyz EDC enables rapid study build with point and click with an interface that helps users build forms and easily navigate to the appropriate screens for data collection and analysis.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content