Generative AI-focused biotech startup Evozyne raises $81m
Pharmaceutical Technology
SEPTEMBER 28, 2023
The company’s algorithms put proteins through millions of years of simulated evolution to identify potential functional candidates.

Pharmaceutical Technology
SEPTEMBER 28, 2023
The company’s algorithms put proteins through millions of years of simulated evolution to identify potential functional candidates.

Bio Pharma Dive
SEPTEMBER 28, 2023
In a note to clients, Evercore ISI analyst Jonathan Miller described the Project NextGen contract for Gritstone as “certainly a nice signal of continued government support for COVID research.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
SEPTEMBER 28, 2023
Speaker Tumaini Rucker Coker, MD, MBA Professor of Pediatrics Division Head for General Pediatrics University of Washington Department of Pediatrics Seattle Children’s Hospital Slides Keywords Pediatrics, Preventive Medicine, Community Health, Well Child Care Key Points There are 10 preventive care visits from ages 0-3, usually scheduled as 15-20 minute visits with a pediatrician.

Bio Pharma Dive
SEPTEMBER 28, 2023
Interim results from a study called “Mariposa” found that a regimen of two J&J medicines improved progression-free survival versus AstraZeneca’s widely used therapy.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharma Times
SEPTEMBER 28, 2023
Collaboration will enable teams across the organisation to increase clinical research innovation - News - PharmaTimes

Bio Pharma Dive
SEPTEMBER 28, 2023
The drug’s success in two late-stage clinical trials has buoyed Karuna to a market valuation exceeding $6 billion.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
SEPTEMBER 28, 2023
The test developer will use the Series B funding to complete a 10,000-person clinical trial ahead of a planned launch in 2025.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Apprentice provides cloud-based software solutions and is a Category Award Winner in the 2023 Pharmaceutical Technology Excellence Awards

Fierce Pharma
SEPTEMBER 28, 2023
Fabre-Kramer Pharmaceuticals' major depressive disorder (MDD) drug gepirone has been rejected by the FDA not once, not twice, but three times since the turn of the millennium. | Fabre-Kramer's Exxua suffered three prior FDA rejections before scoring an FDA approval for major depressive disorder last week. Its label doesn't include sexual dysfunction as an adverse reaction, which is rare among antidepressants.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Bosutinib has been approved for adult use for 10 years, but the FDA has given the green light for use in children.

BioSpace
SEPTEMBER 28, 2023
Pivotal clinical trials in Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis are expected to read out this fall. Here's a closer look.

Pharmaceutical Technology
SEPTEMBER 28, 2023
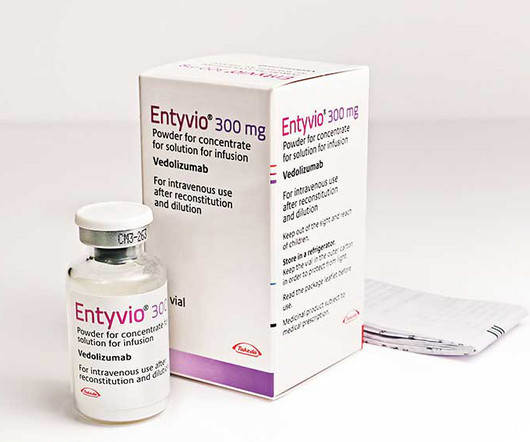
Takeda has received approval from the US FDA for subcutaneous administration of Entyvio for ulcerative colitis in adults.

Fierce Pharma
SEPTEMBER 28, 2023
Slated for an FDA decision last October, Amicus Therapeutics’ Pompe disease bid was foiled by COVID-related travel restrictions. | Slated for an FDA decision last October, Amicus Therapeutics’ Pompe disease bid was foiled by COVID-related travel restrictions. Nearly a year later, the Philadelphia company has gained its long-awaited green light.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Roche signed an agreement attaining global rights to Ionis Pharmaceuticals’ programmes for Alzheimer’s disease and Huntington’s disease.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Fierce Pharma
SEPTEMBER 28, 2023
With much riding on its mRNA patent litigation against BioNTech, Germany’s CureVac thinks the case is moving in its favor. | A court in Germany suspended infringement proceedings on four patents at issue in the lawsuit filed by CureVac against BioNTech. Still, CureVac said there's reason to be optimistic its arguments may win out.
Pharmaceutical Technology
SEPTEMBER 28, 2023
Ono Pharmaceutical and Adimab have signed a drug discovery collaboration agreement for the development of antibody drugs in oncology

pharmaphorum
SEPTEMBER 28, 2023
Understanding and addressing care disparities in rare cancers Mike.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Pharmaceutical Technology has listed some of the leading providers of product inspection, testing and detection equipment and services in the pharmaceutical industry.

Fierce Pharma
SEPTEMBER 28, 2023
While GLP-1 drugs from Novo Nordisk and Eli Lilly are believed to be relatively free of serious side effects, a few problems have emerged as the treatments have gained wider and longer-term use.

Pharmaceutical Technology
SEPTEMBER 28, 2023
It is common for biopharmaceutical companies that start as private entities to become public companies soon after their key pipeline assets reach the clinical stage of development, in order to unlock the large amount of capital needed to run costly clinical trials.
pharmaphorum
SEPTEMBER 28, 2023
CureVac claims advantage in BioNTech patent dispute Phil.

Pharmaceutical Technology
SEPTEMBER 28, 2023
Tetra Therapeutics has received rare paediatric disease designation from the US FDA for zatolmilast to treat Fragile X syndrome (FXS).

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
SEPTEMBER 28, 2023
A highly anticipated head-to-head matchup between a Johnson & Johnson combination and AstraZeneca’s star Tagrisso as a first-line treatment in a subset of non-small cell lung cancer (NSCLC) has | With the trial win, Johnson & Johnson sees potential for its drug combination as the new standard of care in locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer.

Cloudbyz
SEPTEMBER 28, 2023
In the ever-evolving world of clinical research and life-sciences, the efficient and accurate collection of data is paramount. Researchers and clinical trial professionals are constantly seeking innovative solutions to streamline the data collection process, improve data quality, and enhance overall efficiency. Cloudbyz, a leading provider of life sciences technology solutions, has stepped up to the challenge with their state-of-the-art EDC (Electronic Data Capture) platform, combined with the p

pharmaphorum
SEPTEMBER 28, 2023
US channels $104m into antimicrobial resistance project Phil.
Fierce Pharma
SEPTEMBER 28, 2023
The FDA has signed off on Takeda’s subcutaneous version of Entyvio to be used as a maintenance therapy for patients with ulcerative colitis.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Outsourcing Pharma
SEPTEMBER 28, 2023
A study to evaluate the effect of seladelparm, a small molecule treatment by CymaBay Therapeutics, Inc. on patients with cirrhosis was announced this month (September 21).

Fierce Pharma
SEPTEMBER 28, 2023
As bluebird bio’s pricey gene therapy launches take flight, the company is boosting manufacturing capacity with Swiss CDMO Lonza. | Bluebird and Lonza recently amended their production contract for the second time since the deal was announced in summer of 2016. Under the updated deal, Lonza has agreed to increase manufacturing capacity for bluebird’s therapies Zynteglo and Skysona, according to a SEC filing published Wednesday.

XTalks
SEPTEMBER 28, 2023
Fabre Kramer Pharmaceuticals, Inc., a Texas-based pharmaceutical firm specializing in psychotropic drug development, revealed that its groundbreaking antidepressant, Exxua (gepirone extended release) for treating major depressive disorder (MDD), received US Food and Drug Administration (FDA) approval on September 22, 2023. The company initially submitted a New Drug Application (NDA) Amendment to the FDA on December 23, 2022. “Exxua represents an important milestone in the treatment of MDD,

Fierce Pharma
SEPTEMBER 28, 2023
Riding on the growth of long-acting antiretroviral therapy Cabenuva, GSK is laying out a more optimistic vision for its overall HIV business. | Riding on the growth of long-acting antiretroviral therapy Cabenuva, GSK is laying out a more optimistic vision for its overall HIV business.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content