Autobahn raises $100M on investor interest in neuropsych drugs
Bio Pharma Dive
JULY 24, 2024
Autobahn’s lead candidate is designed to stimulate thyroid hormone receptors as a way to complement existing antidepressants.

Bio Pharma Dive
JULY 24, 2024
Autobahn’s lead candidate is designed to stimulate thyroid hormone receptors as a way to complement existing antidepressants.

Pharmaceutical Technology
JULY 24, 2024
The crowd and panel gave Gilead a standing ovation after the full data from the Phase III PURPOSE trial was announced at AIDS 2024.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 24, 2024
We’ve known for over a century that mice and rats live longer when they are fed less, but a new study reveals the secret might be an imbalance between energy consumed and burned, rather than a lack of energy or protein.
Pharmaceutical Technology
JULY 24, 2024
The UK MHRA has approved a new indication of Novo Nordisk’s semaglutide to lower the risk of serious heart problems in overweight adults.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 24, 2024
India’s lower-income groups face significant financial challenges when it comes to cancer treatment. High medical costs, lack of insurance coverage, and limited access to quality healthcare services exacerbate the situation. Many families are seen to opt between basic necessities and treatment, leading to increased mortality rates and a higher burden of disease.

Bio Pharma Dive
JULY 24, 2024
While the study results were positive, questions remain about the longer-term potential of hemophilia treatments like Pfizer’s.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
JULY 24, 2024
The neurodegenerative disease is currently the most common cause of dementia

Bio Pharma Dive
JULY 24, 2024
Top executives at CVS Caremark, Optum Rx and Express Scripts made a rare congressional appearance to defend their companies’ drug pricing policies.

Rethinking Clinical Trials
JULY 24, 2024
In this Friday’s PCT Grand Rounds, Gregg Fonarow of the University of California, Los Angeles, will present “Interventions for Optimization of Guideline-Directed Medical Therapy.” The Grand Rounds session will be held on Friday, July 26, 2024, at 1:00 pm eastern. Fonarow is the Eliot Corday Professor of Cardiovascular Medicine and Science at UCLA, director of the Ahmanson-UCLA Cardiomyopathy Center, and codirector of the UCLA Preventative Cardiology Program.

Bio Pharma Dive
JULY 24, 2024
Known as SAGE-324, the drug was one of the key assets Biogen gained rights to through a billion-dollar research deal inked back in 2020.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Pharma Times
JULY 24, 2024
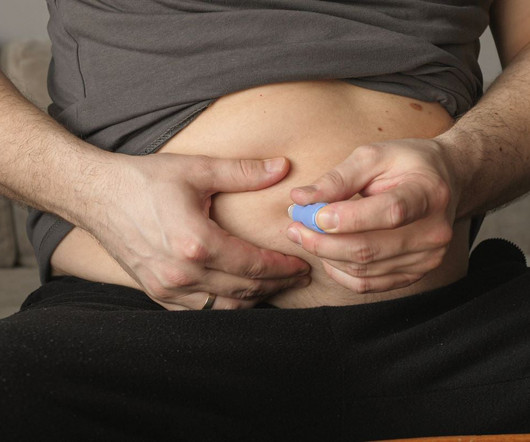
Affecting over 250,000 people, bowel cancer is the fourth most common cancer in the UK

Bio Pharma Dive
JULY 24, 2024
Michelle Tarver, deputy director for transformation, will serve as acting director of the FDA’s medical devices office.
Pharmaceutical Technology
JULY 24, 2024
The Phase II antibody drug conjugate received FDA fast track designation against treatment-resistant head and neck carcinoma.

Bio Pharma Dive
JULY 24, 2024
The Swiss pharma is paying Dren Bio $150 million to partner on “targeted myeloid engagers.” Elsewhere, Geron is losing its commercial chief and Biovectra will sell to Agilent for $925 million.

Pharmaceutical Technology
JULY 24, 2024
3D printing is replacing traditional prototype development approaches across various industries, and use in healthcare is increasing fast.
pharmaphorum
JULY 24, 2024
Stay updated on Matteo Levisetti, the chief medical officer at CUE Biopharma, post ASCO 2024. Learn about his latest research and contributions in the field of biopharma.

Pharmaceutical Technology
JULY 24, 2024
AstraZeneca will pay $45m upfront and up to $500m in milestone-based payments for licencing Pinetree’s preclinical EGFR degrader.

pharmaphorum
JULY 24, 2024
Explore the latest developments in the life sciences industry with a focus on patient-centred research and development (R&D) at DIA 2024. Chart new horizons and stay updated on key trends shaping the future of healthcare.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
JULY 24, 2024
The study found that the risk of certain side effects peak by two weeks for lymphoma patients receiving three CAR-T therapies.

pharmaphorum
JULY 24, 2024
The top three pharmacy benefit managers in the US felt the heat at a hearing of the House Oversight Committee, as lawmakers grilled executives over their business practices and accused them of monopolising the supply chain.

Pharmaceutical Technology
JULY 24, 2024
Telix’s ‘cold kit’ will “improve equity of access” to PSMA-PET imaging, the company’s CEO says.

pharmaphorum
JULY 24, 2024
Asarina Pharma has abandoned its partner search for Tourette's drug sepranolone and will go into liquidation.

Pharmaceutical Technology
JULY 24, 2024
BeiGene has announced the opening of its new US biologics manufacturing and clinical R&D facility at Hopewell, New Jersey.

Pharmaceutical Commerce
JULY 24, 2024
The latest news for pharma industry insiders.

Pharmaceutical Technology
JULY 24, 2024
Merck has entered a non-binding MoU with the Gene Therapy Research Institution (GTRI) to produce a gene therapy for Parkinson's disease.
Drug Patent Watch
JULY 24, 2024
In the high-stakes world of pharmaceuticals, generic drugs have become the unsung heroes of healthcare accessibility.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
JULY 24, 2024
The pharmaceutical industry is facing supply chain challenges, resulting in drug shortages and prompting reorganisation by the pharma sector.

pharmaphorum
JULY 24, 2024
MSD's phase 2b/3 data with clesrovimab for RSV prevention in infants sets up a challenge to Sanofi and AstraZeneca's fast-growing Beyfortus
Pharmaceutical Technology
JULY 24, 2024
Sionna Therapeutic has strengthened its position in the cystic fibrosis (CF) therapeutic landscape through a licensing agreement with AbbVie.

pharmaphorum
JULY 24, 2024
Biogen's $3bn alliance with Sage has had another setback after a drug for essential tremor failed a phase 2 study.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content