Biotech startup Airna raises $60M for RNA editing medicines
Bio Pharma Dive
JULY 31, 2024
Launched last September, the company is working on a treatment for alpha-1 antitrypsin deficiency, which it said could enter the clinic in 2025.

Bio Pharma Dive
JULY 31, 2024
Launched last September, the company is working on a treatment for alpha-1 antitrypsin deficiency, which it said could enter the clinic in 2025.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 31, 2024
The food consumed by a pregnant mother just got a little bit more important. According to a new study on pregnant mice, a diet rich in fiber improves the long-term heart health of developing offspring, significantly lowering their risk of cardiovascular disease later in life.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JULY 31, 2024
Several factors are slowing U.S. sales of Shingrix, the shingles vaccine that's become one of the British pharma's top sellers.

Pharmaceutical Technology
JULY 31, 2024
Pfizer has posted a 98% decrease in net income attributable to shareholders of $41m for Q2 2024, compared to $2.3bn in Q2 2023.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 31, 2024
The Indian Council of Medical Research (ICMR) has announced an Expression of Interest (EoI) inviting eligible organizations, companies, and manufacturers to participate in the ‘Transfer of Technology’ for a CRISPR Cas-based TB detection system.

Pharmaceutical Technology
JULY 31, 2024
MSD has reported a net income on a GAAP basis of $5.4bn for Q2 2024 compared to the net loss of $5.9bn in the same quarter of 2023.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
JULY 31, 2024
Gene therapy research is expensive. Biotechs invest millions of dollars in early research to develop therapies for conditions that might be rare. But when funds are limited and the market potential is dim, such programs are often abandoned by companies, leaving patients and investigators in a bind.

Bio Pharma Dive
JULY 31, 2024
Elsewhere, Fibrogen plans to lay off three-quarters of its U.S. staff, while gene editing specialist Intellia is now cleared to start a U.K. study focused on AAT deficiency.
Pharmaceutical Technology
JULY 31, 2024
The US FDA has granted approval for Johnson & Johnson’s (J&J) DARZALEX FASPRO for treating newly diagnosed multiple myeloma (NDMM).

Pharma Times
JULY 31, 2024
Quantum-based technologies will explore infectious diseases, cancer and dementia

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JULY 31, 2024
The FDA has set a PDUFA target action date of 30 January 2025 for Vertex’s acute pain treatment therapy that is a non-opioid alternative.

Rethinking Clinical Trials
JULY 31, 2024
Dr. Adrian Hernandez In this Friday’s PCT Grand Rounds, Adrian Hernandez of Duke University will present “Precision Health to Population Health: Opportunities and Challenges for Gene Editing Therapies.” The Grand Rounds session will be held on Friday, August 2, 2024, at 1:00 pm eastern. Hernandez is a professor of medicine and vice dean in the Duke University School of Medicine and the executive director of the Duke Clinical Research Institute.

Pharmaceutical Technology
JULY 31, 2024
FibroGen’s pamrevlumab failed to prove its efficacy in two Phase III trials prompting the company to initiate cost-saving measures.

Pharma Times
JULY 31, 2024
The latest collaboration marks Apollo’s sixth agreement with a research institution
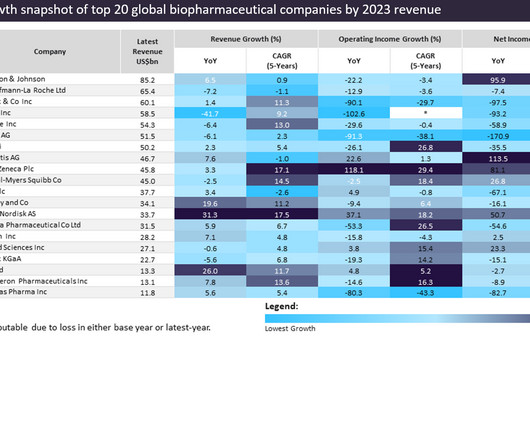
Pharmaceutical Technology
JULY 31, 2024
The biopharmaceutical industry experienced varied revenue shifts in 2023, with significant success for companies with obesity drugs.

pharmaphorum
JULY 31, 2024
Frontiers Health 2024 is a platform connecting innovators and changemakers in the life sciences industry to cultivate positive changes in health systems. Discover the latest trends and insights in healthcare innovation.

Pharmaceutical Technology
JULY 31, 2024
In the intent-to-treat cohort, IMNN-001 plus chemotherapy offered a median 11.1-month increase in overall survival versus chemotherapy alone.

pharmaphorum
JULY 31, 2024
Vertex has filed for FDA approval of its non-opioid pain drug suzetrigine, starting the clock ticking on a review that should be completed early next year

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
JULY 31, 2024
The industry is seeking to scale up and satisfy demand for diabetes and obesity treatments that are now chronically in shortage.
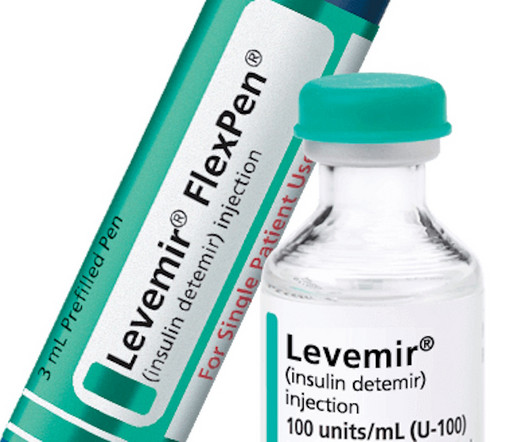
pharmaphorum
JULY 31, 2024
Patient advocacy group APIC has started a campaign to stop Novo Nordisk from following through on a plan to stop making insulin product Levemir

Pharmaceutical Technology
JULY 31, 2024
Leveragen and Moderna have entered a research, option and licence agreement to advance the development of various therapeutics.

pharmaphorum
JULY 31, 2024
Ensuring equitable access to medications remains a fundamental commitment for many pharmaceutical companies to their customers, patients, and stakeholders. However, there has been a discernible shift recently in the timing of access discussions.

Pharmaceutical Technology
JULY 31, 2024
The US Food and Drug Administration application fees have increased by around $300,000 from 2024 and by $2 million compared to a decade ago.
pharmaphorum
JULY 31, 2024
GSK raises 2024 forecasts on a strong Q2 led by HIV and cancer drugs, but its shares weakened on litigation concerns and weaker vaccine expectations

Pharmaceutical Technology
JULY 31, 2024
How many patents did Amgen submit related to internet of things last quarter, and how many were granted? Discover the latest information here.

Fierce Pharma
JULY 31, 2024
Four months after Bayer handed pink slips to 90 staffers at its U.S. headquarters in Whippany, New Jersey, the company is laying off 70 more workers at the site. | Four months after Bayer handed pink slips to 90 staffers at its U.S. headquarters in Whippany, New Jersey, the company is laying off 70 more at the site, according to a state Worker Adjustment and Retraining Notification.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Drug Patent Watch
JULY 31, 2024
By implementing these strategies, you can can enhance your competitiveness in the market while contributing to increased access to affordable […] Source

XTalks
JULY 31, 2024
The US Food and Drug Administration (FDA) has just approved Leqselvi (deuruxolitinib), a new oral medication by Sun Pharmaceutical Industries Limited, offering significant hope for those suffering from severe alopecia areata, a condition causing sudden hair loss. This approval marks a significant milestone, bringing new possibilities to the estimated 300,000 people in the US who deal with severe forms of this autoimmune disease.

Drug Patent Watch
JULY 31, 2024
In the high-stakes world of pharmaceutical development, effective patent portfolio management can mean the difference between blockbuster success and costly […] Source

Pharmaceutical Commerce
JULY 31, 2024
The latest news for pharma industry insiders.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content