Attralus set for amyloidosis asset’s European orphan designations
Pharmaceutical Technology
JULY 29, 2024
EMA’s COMP has adopted positive opinions for two orphan medicinal product designations for Attralus’s AT-02.

Pharmaceutical Technology
JULY 29, 2024
EMA’s COMP has adopted positive opinions for two orphan medicinal product designations for Attralus’s AT-02.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 29, 2024
Social media is full of claims that everyday habits can harm your skin. It’s also full of recommendations or advertisements for products that can protect you. Now social media has blue light from our devices in its sights. So can scrolling on our phones really damage your skin? And will applying creams or lotions help?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 29, 2024
Clinical trial trends point to studying combination therapies and lifestyle changes using alternate designs in Alzheimer’s research.

Bio Pharma Dive
JULY 29, 2024
The deal resembles recent Flagship alliances with Pfizer and Novo Nordisk, allowing the British pharma to comb through the company creator’s portfolio to unearth up to 10 therapies.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JULY 29, 2024
Bristol Myers Squibb (BMS) has posted net income attributable to the company of $1.7bn during Q2 2024, a 19% drop versus $2.1bn in Q2 2023.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 29, 2024
The Indian Council of Medical Research (ICMR), the premier institution for biomedical research in India, has announced a request for Expressions of Interest (EoI) for the establishment of ICMR Collaborating Centres of Excellence (ICMR-CCoE) for the year 2024.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
JULY 29, 2024
Ayman AlAbdallah spoke with PharmaVoice about some of the inroads the sector has made towards a recovery and the traits he looks for in a startup.

Pharmaceutical Technology
JULY 29, 2024
The European agency said that Leqembi’s efficacy was “not large enough” to outweigh its safety risks.

Bio Pharma Dive
JULY 29, 2024
The company claimed “higher than anticipated” scores from trial volunteers on placebo led to the disappointing data, prompting it to halt internal trials.

Pharmaceutical Technology
JULY 29, 2024
Osteoporosis is the most common metabolic bone disorder worldwide and is the leading cause of fragility fractures.

Bio Pharma Dive
JULY 29, 2024
The pharma company will pay up to $1.3 billion to acquire San Diego-based Nerio and its research into novel immune checkpoint inhibitors.

Pharma Times
JULY 29, 2024
Coronary heart disease is the most common form of heart and circulatory disease

Bio Pharma Dive
JULY 29, 2024
The study is the latest test of a class of cholesterol medicines known as CETP inhibitors, which were abandoned by several large drugmakers last decade.
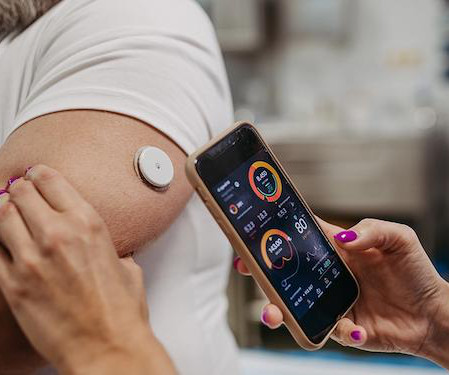
Worldwide Clinical Trials
JULY 29, 2024
With advancements in technology and a deeper understanding of type 2 diabetes (T2D), therapeutics, such as GLP-1 agonists and continuous glucose monitors (CGM), have significantly improved care options for patients with T2D. Despite these advancements, challenges for widespread effective care remain due to the heterogeneous patient population, opening the door for further development of treatments for T2D.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
JULY 29, 2024
The CHMP recommendation is based on positive results from the Phase IIb EFFISAYIL 2 trial which enrolled 123 patients.

Pharma Mirror
JULY 29, 2024
The pharmaceutical industry is constantly shifting, as consumers seek the best treatments. As the market demands new solutions, drugmakers are tasked with investing heavily in research, development, manufacturing and commercialization. Meeting the needs of the sector can be challenging, especially for companies that don’t have the necessary resources.
Pharmaceutical Technology
JULY 29, 2024
The EMA CHMP has recommended approving MSD’s KEYTRUDA regimen as a first-line treatment for urothelial carcinoma.

Fierce Pharma
JULY 29, 2024
Amid an influx of new drugs targeting one of the believed root causes of Alzheimer’s disease, a new symptomatic med has hit the scene, courtesy of Vancouver’s Alpha Cognition. | Amid an influx of new drugs targeting one of the believed root causes of Alzheimer’s disease, a new symptomatic med has hit the scene, courtesy of Vancouver’s Alpha Cognition.

Pharmaceutical Technology
JULY 29, 2024
The European Commission (EC) has granted approval to a €2bn Dutch measure to produce medical radioisotopes used in cancer treatment.

Fierce Pharma
JULY 29, 2024
With a phase 3 trial win claimed by AstraZeneca, the heated BTK inhibitor race might see its first triplet regimen in chronic lymphocytic leukemia (CLL). | With a phase 3 win claimed by AstraZeneca, the heated BTK inhibitor race might see its first triplet regimen in chronic lymphocytic leukemia.
pharmaphorum
JULY 29, 2024
Ipsen gets CHMP backing for Alagille syndrome therapy odevixibat, with a new brand name that it hopes will unlock orphan status in the EU

Fierce Pharma
JULY 29, 2024
Suboxone maker Indivior has spent years dealing with allegations related to its role in the United States opioid epidemic, inking several settlements over that span and even witnessing its former C | More than a dozen states linked up on the agreement after accusing Indivior of fueling the opioid epidemic with its opioid addiction treatment Suboxone.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

pharmaphorum
JULY 29, 2024
Otsuka has teamed up with one of the innovation arms of the NHS to explore how a digital therapeutic (DTx) for major depressive disorder (MDD) could be deployed within the health service.The alliance with Health Innovation Oxford & Thames Valley (Oxford HIN) is focusing on Care for MDD, an app-based DTx that has been co-developed by Otsuka and digital health specialist Click Therapeutics.

Fierce Pharma
JULY 29, 2024
After a prior skirmish over tax policy in England, AstraZeneca is now butting heads with | After a prior skirmish over tax policy in England, AstraZeneca is now butting heads with officials in the country over innovative drug access.

pharmaphorum
JULY 29, 2024
Flagship Pioneering signs another strategic-level deal with a big pharma group, with GSK agreeing an alliance potentially worth more than $7 billion
Outsourcing Pharma
JULY 29, 2024
The Rainwater Charitable Foundation (RCF), in collaboration with CurePSP and the Aging Mind Foundation (AMF), has announced the allocation of $2 million in grants to support groundbreaking research in primary tauopathies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
JULY 29, 2024
NICE and pharma groups AstraZeneca and Daiichi Sankyo have been unable to agree on a price for Enhertu in HER2-low breast cancer.

XTalks
JULY 29, 2024
According to the National Institutes of Health (NIH), over 7,000 identified rare diseases affect an estimated 300 million people worldwide. In fact, the non-profit organization RareX released a report titled The Power of Being Counted in June 2022 that currently identifies the number of recognized rare diseases at 10,867. Despite their individual rarity, the cumulative impact of rare diseases is staggering, with approximately one in 20 people affected by a rare condition at some point in their l

pharmaphorum
JULY 29, 2024
This comprehensive guide provides pharmaceutical companies with valuable insights into biologics contract manufacturing, including the role of CDMOs and key considerations for successful partnerships.

Pharmaceutical Commerce
JULY 29, 2024
A cohort study investigates whether or not the prevalence of these types of illnesses can vary among individuals who are COVID-positive versus negative.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content