FDA approves Galderma’s Nemluvio to treat atopic dermatitis
Pharmaceutical Technology
DECEMBER 16, 2024
The US FDA has approved Galderma's Nemluvio (nemolizumab), for individuals aged 12 years and above with moderate-to-severe atopic dermatitis.

Pharmaceutical Technology
DECEMBER 16, 2024
The US FDA has approved Galderma's Nemluvio (nemolizumab), for individuals aged 12 years and above with moderate-to-severe atopic dermatitis.

AuroBlog - Aurous Healthcare Clinical Trials blog
DECEMBER 16, 2024
Children with attention deficit hyperactivity disorder ( ADHD) do not have a behavioural disorder, nor are they lazy, or lacking in manners and boundaries. Their brains mature in a different way, with different patterns of neurological activity and a number of neurochemical differences. For this reason, ADHD is considered to be a neurodevelopmental disorder.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
DECEMBER 16, 2024
Manufacturing practices are evolving to advance antibody drug conjugates (ADCs) for cancer treatment through a targeted approach.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharma Mirror
DECEMBER 16, 2024
CPHI Milan the worlds largest pharma event hosted last month in Milan saw a new record attendance of 59,000 pharma executives buoyed by returning confidence and increased deal-making on site. The post Pharma prepares for CDMO growth in 2025 as CPHI Milan reports record attendance appeared first on Pharma Mirror Magazine.

Pharmaceutical Technology
DECEMBER 16, 2024
The biopharmaceutical industry faces a future full of risks and rewards. But what role will 3PL suppliers play?
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
DECEMBER 16, 2024
New HTA statistics produced by Denmarks Medicinrdet for 2024 have prompted GlobalData to assess evolving HTA trends in the Nordic market.

Bio Pharma Dive
DECEMBER 16, 2024

Pharmaceutical Technology
DECEMBER 16, 2024
Boehringer Ingelheims blockbuster Ofev could be set for an expanded indication in children and adolescents with fibrosing ILDs.
Pharmaceutical Technology
DECEMBER 16, 2024
Discover the latest advancements in small cell lung cancer through Novotech CROs in-depth disease analysis.

Pharmaceutical Technology
DECEMBER 16, 2024
WuXi AppTec and WuXi Biologics are looking to sell off sites in the US and Europe as the prospect of the Biosecure Act is deterring potential clients.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
DECEMBER 16, 2024
The EMA's CHMP has recommended Lillys Omvoh (mirikizumab) for approval in the EU to treat Crohn's disease.

Bio Pharma Dive
DECEMBER 16, 2024

Pharmaceutical Technology
DECEMBER 16, 2024
AbbVie has entered into a definitive agreement to acquire Nimble Therapeutics in a deal valued at $200m in cash.

Fierce Pharma
DECEMBER 16, 2024

Pharma Times
DECEMBER 16, 2024
New treatment could revolutionise care for HAE patients

Pharma Times
DECEMBER 16, 2024
Phase 2a study shows promising antidepressant effects

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
DECEMBER 16, 2024
XTalks
DECEMBER 16, 2024
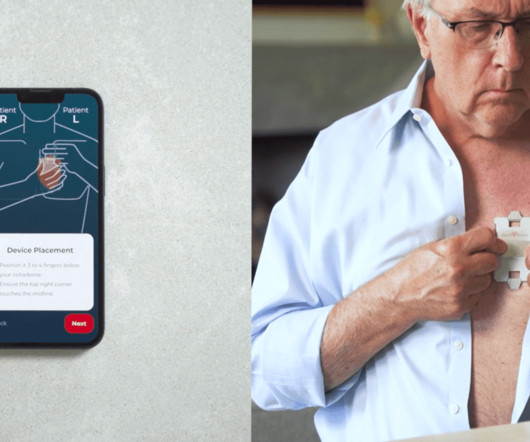
Imagine having a heart monitoring device small enough to fit in a purse or a wallet, ready to record critical heart data whenever symptoms arise. This device by HeartBeam, Inc. is a breakthrough in at-home cardiac care, allowing patients to capture high-quality electrocardiograms (ECGs) wherever they are. When symptoms occur, patients simply place the device on their chest and follow prompts from a companion app.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

XTalks
DECEMBER 16, 2024
Maria Hopfgarten Head of Global Medical Writing PPD Clinical Research Business of Thermo Fisher Scientific Artificial intelligence (AI) is revolutionizing industries worldwide, and medical writing is no exception. With the integration of human intelligence (HI) with AI, the field is discovering new efficiencies and maintaining, or even improving, the rigor required for quality and compliance.

Fierce Pharma
DECEMBER 16, 2024

Pharmaceutical Commerce
DECEMBER 16, 2024
A panel examines the gross-to-net bubble across various product mixes, how pricing impacts market access, and more.

Fierce Pharma
DECEMBER 16, 2024

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content