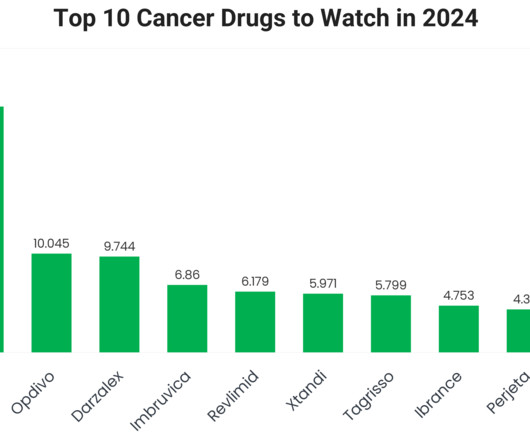
Top 10 Cancer Drugs to Watch in 2024 Based on Recent Sales Data
XTalks
NOVEMBER 26, 2024
As oncology continues to dominate the pharmaceutical market, the global demand for effective cancer therapies has reached unprecedented levels. With advances in precision medicine and immunotherapy driving innovation, leading oncology drugs are not only transforming treatment paradigms but also generating billions in revenue. The year 2023 was no exception, with several cancer drugs achieving remarkable sales milestones, reflecting their efficacy, expanding indications and the growing global can












































Let's personalize your content