FDA denies expanded approval for Alnylam RNA drug
Bio Pharma Dive
OCTOBER 9, 2023
The agency rejected Alnylam’s application for approval of its medicine patisiran in people with a rare heart condition, setting back the company’s plans.

Bio Pharma Dive
OCTOBER 9, 2023
The agency rejected Alnylam’s application for approval of its medicine patisiran in people with a rare heart condition, setting back the company’s plans.

Pharmaceutical Technology
OCTOBER 9, 2023
GSK and Chongqing Zhifei have signed an exclusive agreement for co-promoting the former’s shingles vaccine, Shingrix, in China.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 9, 2023
The purchase agreement with Chongqing Zhifei Biological Products is part of GSK’s plan to double sales of the shingles vaccine by 2026.
Pharmaceutical Technology
OCTOBER 9, 2023
Diabetes researchers oppose the regulation of islet transplantation as a biologic, following the approval of CellTrans’ Lantidra.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 9, 2023
Getting up at least 50 steps a day is associated with a more than 20 percent cut in the risk of cardiovascular disease, new research shows – so you may only need to climb five flights of stairs per day to significantly boost your heart health.

Rethinking Clinical Trials
OCTOBER 9, 2023
The NIH Pragmatic Trials Collaboratory will offer a full-day workshop at the 16th Annual Conference on the Science of Dissemination and Implementation in Health in Arlington, Virginia. The workshop, “Dissemination & Implementation in Embedded Pragmatic Trials: Raising the Bar for Real-World Research,” will introduce concepts in the design, conduct, and implementation of pragmatic clinical trials embedded in healthcare systems, with a particular focus on methods relevant to health
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
OCTOBER 9, 2023
Oncology manufacturers can improve access by using claims, labs, pathway and policy data to address misalignment.

Pharmaceutical Technology
OCTOBER 9, 2023
More than 20 pharmaceutical companies across the UK have hit out against a proposed measure in the UK's regulation of medicines.

Fierce Pharma
OCTOBER 9, 2023
Following similar initiatives to bolster mRNA vaccine production and access in Africa, the Bill & Melinda Gates Foundation is providing new funding to a clutch of drugmakers. | Following similar initiatives to bolster mRNA vaccine production and access in Africa, the Bill & Melinda Gates Foundation is providing new funding to a clutch of drugmakers.

Pharmaceutical Technology
OCTOBER 9, 2023
The approval marks the first intravenous (IV) formulation treatment in adults with rheumatic diseases in six years.

Pharma Times
OCTOBER 9, 2023
The agreement builds on the companies’s existing partnership - News - PharmaTimes

Pharmaceutical Technology
OCTOBER 9, 2023
Bristol Myers Squibb (BMS) signed a definitive agreement to acquire all outstanding shares of Mirati Therapeutics for $5.8bn.

Pharma Times
OCTOBER 9, 2023
The study will offer new insights into the immune system's response to iGAS - News - PharmaTimes

Pharmaceutical Technology
OCTOBER 9, 2023
Semaglutide: compared to key European markets, its higher price and that of other diabetes/obesity drugs in the US could impact on healthcare

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
OCTOBER 9, 2023
Alongside diagnosis: AI’s role in shaping health transformation outcomes Mike.

Pharmaceutical Technology
OCTOBER 9, 2023
The FDA decision follows a similar designation for the vaccine UV1 against malignant melanoma in December 2021.

Fierce Pharma
OCTOBER 9, 2023
GSK has forged a partnership with vaccine powerhouse Chongqing | GSK has forged a partnership with vaccine powerhouse Chongqing Zhifei Biological Products to distribute Shingrix in China. Zhifei will pay £2.5 billion ($3.05 billion) for exclusive rights to distribute the shingles shot in China from 2024 through 2026. The deal could be a precursor to similar arrangement between the companies for GSK’s new respiratory syncytial virus (RSV) vaccine Arexvy, the London-based drugmaker said.

Pharmaceutical Technology
OCTOBER 9, 2023
The FDA CRL cited insufficient evidence to approve the use of Onpattro for treating cardiomyopathy of ATTR amyloidosis.

pharmaphorum
OCTOBER 9, 2023
Curio DTx hits mark in postpartum depression trial Phil.

Pharmaceutical Technology
OCTOBER 9, 2023
The Series A funds will help advance a pipeline, which includes drugs that provide both symptomatic and disease-modifying benefits.

Drug Patent Watch
OCTOBER 9, 2023
The article titled “Reviving an R&D Pipeline: A Step Change in the Phase II Success Rate” discusses Pfizer’s efforts to improve its research and development (R&D) productivity. The pharmaceutical industry… The post Reviving an R&D Pipeline appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
OCTOBER 9, 2023
Janssen has submitted an application seeking approval from the EMA for the NSCLC combo therapy, RYBREVANT plus chemotherapy.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
OCTOBER 9, 2023
Roche preps for filings after subcutaneous Ocrevus matches up to IV formulation in phase 3 study zbecker Mon, 10/09/2023 - 18:07

Pharmaceutical Technology
OCTOBER 9, 2023
Amgen has acquired Horizon Therapeutics in a deal valued at $27.8bn following approval from the US Federal Trade Commission.

Fierce Pharma
OCTOBER 9, 2023
Despite an endorsement from an advisory committee, the FDA has come to a different conclusion on the benefit-risk profile for Alnylam’s Onpattro in the rare heart disease transthyretin amyloidosis | Despite an endorsement from an advisory committee, the FDA has come to a different conclusion on the benefit-risk profile for Alnylam’s Onpattro in the rare heart disease transthyretin amyloidosis cardiomyopathy (ATTR-CM).

Pharmaceutical Technology
OCTOBER 9, 2023
Limmatech, a GSK spinoff focused on combatting antimicrobial resistance, had sought series A funding seven years after foundation.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
OCTOBER 9, 2023
BMS swoops on Mirati with $5.8bn takeover offer Phil.
Drug Patent Watch
OCTOBER 9, 2023
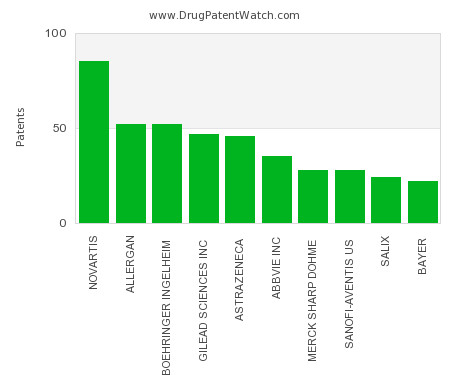
This chart shows the pharmaceutical companies with the most patents in Mexico. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is… The post Which pharmaceutical companies have the most drug patents in Mexico? appeared first on DrugPatentWatch - Make Better Decisions.
pharmaphorum
OCTOBER 9, 2023
Scotland first in Europe to back MSD drug for VHL tumours Phil.

Fierce Pharma
OCTOBER 9, 2023
The way we consume and engage with content is changing all the time. | How can marketers leverage AI to make sense of the masses of data they have access to? Chris Paquette, Founder and CEO of DeepIntent, explores the issues of fragmentation and outlines some of the tools designed to overcome it.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content