BridgeBio sends rare disease drugs to new company
Bio Pharma Dive
AUGUST 21, 2024
Backed by $300 million from investors that include Viking Global Investors and Sequoia Capital, GondolaBio will inherit several BridgeBio drug programs.

Bio Pharma Dive
AUGUST 21, 2024
Backed by $300 million from investors that include Viking Global Investors and Sequoia Capital, GondolaBio will inherit several BridgeBio drug programs.

Pharmaceutical Technology
AUGUST 21, 2024
Amgen has announced the availability of Otezla (apremilast) in the US for paediatric patients with moderate to severe plaque psoriasis.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
AUGUST 21, 2024
Pharmaceuticals like the in-demand GLP-1 drugs for obesity are expected to drive increases in healthcare spending in 2025, per a new employer survey.

Pharmaceutical Technology
AUGUST 21, 2024
The China NMPA approved AstraZeneca's Fasenra for individuals aged 12 years and older with severe eosinophilic asthma (SEA).

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
AUGUST 21, 2024
Company executives had previously warned investors that a delay was likely due to issues at a facility run by third-party manufacturer.

Pharmaceutical Technology
AUGUST 21, 2024
The deal, valued at approximately $30m, contributes to Emergent’s financial recovery plan amid its dramatic post-Covid decline.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
AUGUST 21, 2024
Dr Reddy's Laboratories, Aurigene Pharmaceutical and Kainomyx signed an MoU to co-develop and co-commercialise an anti-malarial drug.

Bio Pharma Dive
AUGUST 21, 2024
Greg Friberg and James Sabry will take over, respectively, as heads of R&D and business development, less than a year after BioMarin named a new CEO.
Pharma Times
AUGUST 21, 2024
The new test takes an average of 13 hours to identify the correct treatment compared to several days with current methods
Pharmaceutical Technology
AUGUST 21, 2024
The funds will support Pathalys’ lead candidate upacicalcet through two clinical trials and potentially accelerate its approval.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Pharma Times
AUGUST 21, 2024
Globally 400,000 children are impacted every year by cancers that include leukaemia and lymphoma

Pharmaceutical Technology
AUGUST 21, 2024
The issue at a third-party manufacturer has been resolved, with an FDA re-inspection now planned.

Rethinking Clinical Trials
AUGUST 21, 2024
In this Friday’s PCT Grand Rounds, David Bekelman and Lyndsay DeGroot of the University of Colorado will present “Improving Quality of Life in COPD and Heart Failure: Unpacking a Successful Multicomponent Virtual Team Intervention.” The Grand Rounds session will be held on Friday, August 23, 2024, at 1:00 pm eastern. Bekelman is a professor of medicine and psychiatry at the University of Colorado School of Medicine and the Rocky Mountain Regional VAMC.

Pharmaceutical Technology
AUGUST 21, 2024
Pharma companies seeking to commercialise in mRNA can gain a competitive edge by partnering with expert suppliers

pharmaphorum
AUGUST 21, 2024
Demonstrating EVERSANA's dedication to innovation, a novel artificial intelligence technology is set to enhance the NAVLIN experience for both current and prospective customers.
Pharmaceutical Technology
AUGUST 21, 2024
New research has recently demonstrated how contraception for women can be personalised based on genetics and individual needs.

pharmaphorum
AUGUST 21, 2024
J&J's Rybrevant/Lazcluze combination is cleared by FDA for first-line EGFR+ NSCLC, giving AstraZeneca's top-seller Tagrisso its first direct competition
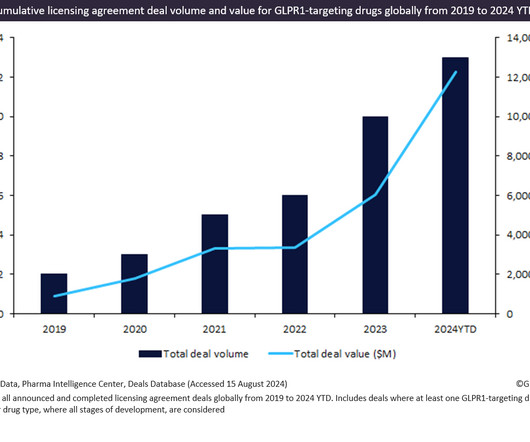
Pharmaceutical Technology
AUGUST 21, 2024
Innovator drugs targeting GLP1R witnessed a 595% increase in total licensing agreement deal value from 2019 to 2024 YTD.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
XTalks
AUGUST 21, 2024
The US Food and Drug Administration (FDA) has approved Voranigo (vorasidenib) for Grade 2 IDH-mutant glioma, a challenging brain tumor that has seen limited effective treatments for nearly 25 years. Developed by Servier Pharmaceuticals, Voranigo offers the first targeted therapy for patients 12 years and older with Grade 2 astrocytoma or oligodendroglioma that harbor isocitrate dehydrogenase-1 (IDH1) or isocitrate dehydrogenase-2 (IDH2) mutation.

Pharmaceutical Technology
AUGUST 21, 2024
The company halted Phase II trial of its mTOR inhibitor after an independent analysis found that it would not meet the efficacy threshold.

pharmaphorum
AUGUST 21, 2024
Debate over the safety of GLP-1 drugs resurfaces after study finds 'disproportional' risk of suicidal thinking adverse event reports with Novo Nordisk's semaglutide

Pharmaceutical Technology
AUGUST 21, 2024
Organon and Eli Lilly broadened a commercial agreement under which the former will distribute and promote Emgality in 11 new markets.

pharmaphorum
AUGUST 21, 2024
A class action suit has been filed in the US against Teva, claiming that the company illegally subsidised the copays for patients prescribed its multiple sclerosis therapy Copaxone to boost its sales.The suit filed on behalf of Medicare Advantage coverage providers accuses the company of "funnelling hundreds of millions of dollars" to Copaxone (glatiramer acetate) patients through third-party foundations and companies.

Fierce Pharma
AUGUST 21, 2024
As Biogen’s relentless patent defense for blockbuster multiple sclerosis med Tecfidera came up short in the U.S., the company separately worked to fend off competition through an anticompetitive ki | A multi-employer health benefit plan in Illinois accused the drugmaker of scheming with pharmacy benefit managers to keep its Tecfidera in a more favorable spot on their formularies.

pharmaphorum
AUGUST 21, 2024
Digital technologies play a crucial role in helping the pharmaceutical industry meet the changing needs of healthcare providers (HCPs) and patients. Learn how digital innovations are transforming healthcare delivery and improving patient outcomes in this comprehensive guide.

Fierce Pharma
AUGUST 21, 2024
A survey of 800 biopharma executives shows that sustainability is an increasingly important priority but that companies are struggling to achieve it.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

BioPharma Reporter
AUGUST 21, 2024
In a groundbreaking development, a team of biochemical engineers from South Korea has unveiled a new exosome-based delivery system, termed MAPLEX, that holds the potential to transform treatments for various diseases, including Alzheimerâs.

Fierce Pharma
AUGUST 21, 2024
A year after missing on a trial endpoint, Travere Therapeutics can breathe a sigh a relief. | A year after missing on a trial endpoint, Travere Therapeutics can breathe a sigh a relief. The FDA has converted Filspari’s conditional nod in the kidney disease IgAN into a full approval but left a liver toxicity monitoring requirement in place.
BioPharma Reporter
AUGUST 21, 2024
A team of scientists from the universities of Glasgow and Tel Aviv has developed a groundbreaking method to transform the brain parasite Toxoplasma gondii into a potential vehicle for delivering therapeutic treatments directly to brain cells.
pharmaphorum
AUGUST 21, 2024
The Tony Blair Institute (TBI) has called for a digital health record (DHR) for everyone in the UK within five years to "ensure that the NHS is ready for the artificial intelligence era."The DHR would serve as a "single source of truth" for health and care data that is currently fragmented across multiple NHS sources and phones, and serve as a "building block" for the way health will be delivered in future, according to the think tank.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content