Tech leader’s $125m boosts Parker Institutes immunotherapy research
Pharmaceutical Technology
JULY 19, 2024
The Parker Institute for Cancer Immunotherapy was founded in 2016 by tech entrepreneur Sean Parker to bolster cancer research efforts.

Pharmaceutical Technology
JULY 19, 2024
The Parker Institute for Cancer Immunotherapy was founded in 2016 by tech entrepreneur Sean Parker to bolster cancer research efforts.

Bio Pharma Dive
JULY 19, 2024
Federal filings showed no companies other than Lilly made an offer to buy Morphic, while Novartis executives said they’re still waiting for more data on pelabresib.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JULY 19, 2024
New global projections and advances in HIV therapies are also on the agenda at the 25th International AIDS 2024 meeting.

Bio Pharma Dive
JULY 19, 2024
At least 11 health systems were experiencing issues Friday after an update by cybersecurity firm CrowdStrike went awry.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!
Pharma Times
JULY 19, 2024
LT-HSCs replenish blood cells after treatment for blood cancers, such as leukaemia

Pharmaceutical Technology
JULY 19, 2024
The Opdivo/Yervoy combination demonstrated statistically and clinically significant improvements in the Phase III CheckMate-9DW study.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
JULY 19, 2024
Exscientia paid $20m in upfront cash and equity to buy its development partner GT Apeiron’s shares for the CDK7 inhibitor.

Pharma Times
JULY 19, 2024
Accreditation recognises 'continued professional development' opportunities

Pharmaceutical Technology
JULY 19, 2024
Boehringer Ingelheim will sell an unbranded copycat of Humira through GoodRx for $550 in a two- pack form.

pharmaphorum
JULY 19, 2024
FDA will create a rare disease hub to speed the development of new treatments and build connections between developers and the rare disease community

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JULY 19, 2024
Rona Therapeutics has secured $35m in its Series A+ financing round to advance its metabolic siRNA pipeline in clinical settings.

pharmaphorum
JULY 19, 2024
Explore the emerging era of digital health and pharmaceuticals with PDURS, featuring insights from industry experts Marty Culjat of EVERSANA and Edward Cox of Pfizer.
Pharmaceutical Technology
JULY 19, 2024
The EMA has accepted the MAA of Deciphera Pharmaceuticals' vimseltinib to treat tenosynovial giant cell tumours for review.
pharmaphorum
JULY 19, 2024
Explore the emerging uses and future directions of chimeric antigen receptor T-cell therapy targeting mRNA, anti-EGFRvIII, and anti-NY-ESO-1 antigens in cancer treatments.

Pharmaceutical Technology
JULY 19, 2024
Meitheal Pharmaceuticals has gained rights to CONTEPO (fosfomycin for injection) from Nabriva Therapeutics for the North American region.
Pharmaceutical Commerce
JULY 19, 2024
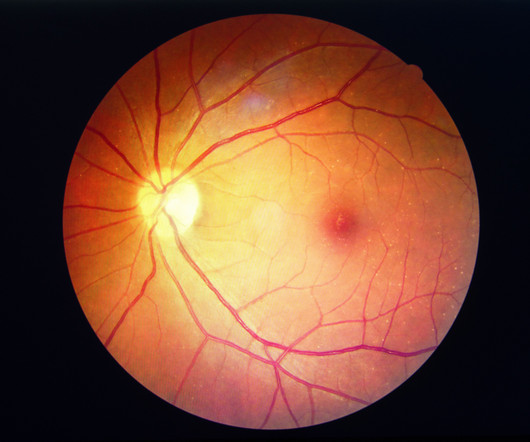
FDA to review one-year results for Susvimo showing non-inferior visual acuity gains in diabetic macular edema and in the Diabetic Retinopathy Severity Scale for Diabetic Retinopathy.

pharmaphorum
JULY 19, 2024
NICE backs NHS use of Accord's Orgovyx, the first therapy for advanced hormone-sensitive prostate cancer that can be taken at home

XTalks
JULY 19, 2024
Oticon Medical announced that the US Food and Drug Administration (FDA) cleared its first active transcutaneous bone conduction hearing system, the Sentio System. This innovative system offers the benefits of the Ponto System with a new transcutaneous option for patients. Bone conduction hearing aids amplify sound via vibrations through the bones of the skull, directly stimulating the cochlea without needing any part of the device in the ear canal.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
JULY 19, 2024
While the pace of biotech private rounds seems to have slowed a little of late, this week saw four big financings for Cardurion Pharma, Scorpion Therapeutics, CatalYm, and NGM Bio.Burlington, Massachusetts-based Cardurion led the quartet with a $260 million Series B for its heart-focused drug pipeline, which features two candidates in phase 2 testing – PDE9 inhibitor CRD-750 for heart failure and CaMKII inhibitor CRD-4730 for rare genetic disease catecholaminergic polymorphic ventricular tachyca

XTalks
JULY 19, 2024
According to a recent report from LegitScript, a leading provider of healthcare product verification and monitoring services, there has been a whopping 1200 percent increase in violative and misleading advertisements related to compounded GLP-1 medications. Compounded GLP-1 medications, created by specialized pharmacies under a special US Food and Drug Administration (FDA) allowance due to ongoing shortage, have become increasingly popular for weight loss.
pharmaphorum
JULY 19, 2024
Less than two years after pulling multiple myeloma therapy Blenrep off most markets around the world, GSK has filed for approval of the drug in the EU once again, hoping to restore its blockbuster credentials.The remarkable renaissance for the BCMA-directed antibody-drug conjugate is supported by a pair of phase 3 trials that could not only support its return but also make it an option for a much larger patient population than before.

Outsourcing Pharma
JULY 19, 2024
Avista, a New York-based private equity firm focused on healthcare, and Hamilton Lane, a global private markets investment management firm, have announced that funds managed by Hamilton Lane have acquired a significant equity interest in Cosette Pharmaceuticals from Avista and its co-investors.

XTalks
JULY 19, 2024
The term “daily value” is central to understanding the Nutrition Facts label, a staple on nearly every packaged food product in the US since 1994. Introduced to enhance consumer transparency, this label has evolved into a model for various industries, promoting socially responsible markets through intuitive information dissemination. The history and implementation of the Nutrition Facts label are more complex than they appear.

Fierce Pharma
JULY 19, 2024
While the national IT outrage caused by cybersecurity specialist CrowdStrike—and subsequently Microsoft—has grounded airlines and forced cancelations at hospitals, the experience from the meltdown | While the national IT outrage caused by cybersecurity specialist CrowdStrike—and subsequently Microsoft—has grounded airlines and forced cancelations at hospitals, the experience from the meltdown appeared different among large pharma companies.

Outsourcing Pharma
JULY 19, 2024
Phathom Pharmaceuticals, Inc.dedicated to developing and commercializing treatments for gastrointestinal (GI) diseases, announced today that the US Food and Drug Administration (FDA) has approved Voquenza (vonoprazan) 10mg tablets for the relief of heartburn associated with non-erosive gastroesophageal reflux disease (non-erosive GERD) in adults.

Fierce Pharma
JULY 19, 2024
A 10-page Form 483 has detailed the shortfalls at Hengrui Pharma's PD-1 plant behind a recent FDA rejection. Sanofi is investing heavily to expand its global capacity center in India. | A 10-observation Form 483 has detailed the shortfalls at Hengrui Pharma's PD-1 plant behind its recent FDA rejection. Sanofi is investing heavily to expand its global capacity center in India.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Outsourcing Pharma
JULY 19, 2024
Phathom Pharmaceuticals, Inc.dedicated to developing and commercializing treatments for gastrointestinal (GI) diseases, announced today that the US Food and Drug Administration (FDA) has approved Voquezna (vonoprazan) 10mg tablets for the relief of heartburn associated with non-erosive gastroesophageal reflux disease (non-erosive GERD) in adults.
Fierce Pharma
JULY 19, 2024
Back in February, Novo Holdings laid out a $16.5 billion deal to snap up contract manufacturing giant Catalent. | In February, Novo Holdings announced a $16.5 billion deal to acquire Catalent, and this week on "The Top Line," we explore the current state and implications of the proposed buyout.

Drug Patent Watch
JULY 19, 2024
This chart shows the pharmaceutical companies with the most pellets dosed drugs. For a different perspective, see the most popular dosage types.

XTalks
JULY 19, 2024
The US Food and Drug Administration (FDA) is stepping up to tackle the complexities of rare diseases, with the new Rare Disease Innovation Hub. This new initiative aims to speed up treatment and care for over 30 million Americans living with these conditions, focusing on enhancing outcomes and accelerating the approval of innovative therapies. Rare diseases affect approximately one in 10 Americans, with children making up nearly half of this demographic.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content