Pfizer quits Duchenne gene therapy, lays off staff following study setback
Bio Pharma Dive
JULY 30, 2024
The company is letting go of 150 staffers alongside a decision to officially terminate the high-profile program, which was acquired in 2016.

Bio Pharma Dive
JULY 30, 2024
The company is letting go of 150 staffers alongside a decision to officially terminate the high-profile program, which was acquired in 2016.

Pharmaceutical Technology
JULY 30, 2024
Semaglutide, marketed as Ozempic and Wegovy, decreased tobacco-related healthcare encounters compared to other medications.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 30, 2024
When viruses pay us a visit, they sometimes leave parts of themselves behind. Silently tucked away in our genomes, some of these bits of foreign DNA can get passed down through the generations.

Pharmaceutical Technology
JULY 30, 2024
GSK has announced a strategic collaboration with Flagship Pioneering for the discovery and development of medicines and vaccines.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
JULY 30, 2024
The CEO noted how Pfizer's danuglipron could still be the second oral GLP-1 treatment to enter registrational tests after Lilly's orforglipron.

Pharmaceutical Technology
JULY 30, 2024
Collegium will pay $525m upfront to add Ironshore’s FDA-approved ADHD medication Jornay PM to its portfolio.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
JULY 30, 2024
Ventyx no longer plans to conduct additional clinical trials of VTX958 with internal resources after the Phase II trial missed its primary endpoint.
Pharma Times
JULY 30, 2024
The rapidly progressive neurological condition affects around 5,000 people in the UK
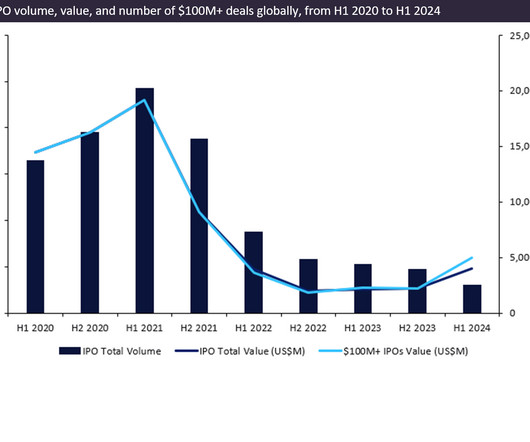
Pharmaceutical Technology
JULY 30, 2024
Biotech IPOs surged in Q1 2024, with eight completed IPOs raising a total of $3.72bn, six times more than the $621m raised in Q4 2023.

Rethinking Clinical Trials
JULY 30, 2024
The Health Care Systems Research Network (HCSRN) will hold its 2025 Annual Conference from April 8 to 10, 2025, in St. Louis, Missouri. HCSRN is a 20-member research network focused on supporting research institutes aligned with healthcare delivery systems. HCSRN’s mission is to improve individual and population health through research that connects the resources and capabilities of learning healthcare systems.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
JULY 30, 2024
The European Commission (EC) has approved Vabysmo (faricimab) for the treatment of retinal vein occlusion.

Bio Pharma Dive
JULY 30, 2024
The company will stop developing five experimental treatments, including two oral checkpoint inhibitors targeting PD-L1.

Pharmaceutical Technology
JULY 30, 2024
Boehringer Ingelheim has acquired Nerio Therapeutics, a drug discovery and development company, in a deal valued at $1.3bn.

Antidote
JULY 30, 2024
In the latest installment of Talk of the Towne, we sat down with Melanie Paris, M.A., MPH, the Senior Director of Strategic Partnerships and Kidney Disease Education at the American Kidney Fund (AKF). In the discussion, we got her insights into the types of kidney disease, treatment disparities, and the importance of clinical research in the field. She also shared many valuable resources for individuals and their loved ones living with kidney disease, which are linked here.

Pharmaceutical Technology
JULY 30, 2024
The UK NICE has decided not to recommend Daiichi Sankyo and AstraZeneca’s Enhertu for advanced HER2-low breast cancer for NHS use.

pharmaphorum
JULY 30, 2024
Discover how OPEN Health and fusion's AI technology partnership is creating a symbiotic relationship that is revolutionising healthcare. Explore the benefits of this for optimal patient care.
Pharmaceutical Technology
JULY 30, 2024
The US FDA has granted priority review designation for Novartis’ Scemblix for Ph-positive chronic myeloid leukemia in the chronic phase.

pharmaphorum
JULY 30, 2024
Boehringer buys Nerio Therapeutics for $1.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
JULY 30, 2024
Trials and their partners need to adopt a strong cybersecurity strategy to protect sensitive patient data from hackers.

pharmaphorum
JULY 30, 2024
Implementing strong cybersecurity guidelines for remote monitoring in clinical trials is crucial to protect sensitive data. Learn these top 5 guidelines to secure your remote monitoring plan effectively.

Fierce Pharma
JULY 30, 2024
For more than a year after Pfizer’s COVID product sales peaked at the end of 2022, the company’s quarterly revenues showed year-over-year declines. | For the first time since its sales peaked during the pandemic, Pfizer registered year-over-year revenue growth during the second quarter of 2024. And while the busy has been busy trimming operating expenses, it did add $1 billion to its total revenue projection for the year.

pharmaphorum
JULY 30, 2024
A small study of Novo Nordisk's once-daily injectable GLP-1 agonist liraglutide shows a benefit in patients with Alzheimer's disease

Pharmaceutical Commerce
JULY 30, 2024
The latest news for pharma industry insiders.

pharmaphorum
JULY 30, 2024
In a new pharmaphorum podcast, web editor Nicole Raleigh speaks with Ethan Drower, co-founder and CEO of Cite Medical Solutions, a company providing full Clinical Evaluation Report (CER) services and industry literature review for European Union Medical Device Regulation (or EU MDR) processes.

Drug Patent Watch
JULY 30, 2024
The Power of Patent Licensing: Unlocking Innovation and Access to Medicines Patent licensing agreements have become a crucial tool in the pharmaceutical industry, allowing companies to collaborate and…

BioPharma Reporter
JULY 30, 2024
A book, a computer and some sage words from her physicist father set Tehmina on her path to a successful career in science.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

pharmaphorum
JULY 30, 2024
mRNA specialist BioNTech has chalked up an important win in its cancer pipeline, showing efficacy with its BNT111 vaccine candidate in a phase 2 trial in advanced melanoma.The study is looking at the combination of BNT111 with Regeneron's PD-1 inhibitor Libtayo (cemiplimab) in 184 patients with advanced melanoma who have relapsed after or not responded to treatment with a PD-1/PD-L1 inhibitor, or the two therapies given alone.
BioSpace
JULY 30, 2024
The advantages of using circular RNAs—including increased durability, enhanced protein expression, and substantially lower manufacturing costs compared to linear mRNAs—have driven a spate of investment in this technology.

XTalks
JULY 30, 2024
Salmonella control in poultry in the US has come under intense regulatory scrutiny over the past few years. With increasing concerns about food safety, it might be worth considering whether the US should adopt the European Union’s (EU) regulatory mindset. The US Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) declared Salmonella an adulterant in raw breaded stuffed chicken products when contamination levels exceed one colony-forming unit (CFU) per gram.
BioPharma Reporter
JULY 30, 2024
In the rapidly evolving field of pharmaceuticals, the development of parenteral drugs and bioconjugates presents unique challenges and opportunities.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content