Study reveals AI insights can predict development of disease a decade in advance
Pharma Times
AUGUST 8, 2024
The study predicted the development of conditions including Alzheimer’s and heart disease

Pharma Times
AUGUST 8, 2024
The study predicted the development of conditions including Alzheimer’s and heart disease

Bio Pharma Dive
AUGUST 8, 2024
Sales of the in-demand obesity drug crested $1 billion in the second quarter as improved Lilly production helped the company fulfill wholesaler backorders.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
AUGUST 8, 2024
Novartis has secured US Food and Drug Administration (FDA) accelerated approval for Fabhalta (iptacopan) to treat igAN.

Bio Pharma Dive
AUGUST 8, 2024
After months of supply strains, Lilly has begun to catch up with demand. Consumers may still need to wait for their prescriptions to be filled, however.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Pharmaceutical Technology
AUGUST 8, 2024
Conduit plans to advance two assets, AZD1656 and AZD5658, into Phase II testing for autoimmune disorders.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 8, 2024
Last week, the Australian Therapeutic Goods Administration added intravenous (IV) fluids to the growing list of medicines in short supply. The shortage is due to higher-than-expected demand and manufacturing issues. Two particular IV fluids are affected: saline and compound sodium lactate (also called Hartmann’s solution). Both fluids are made with salts.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 8, 2024
The Indian healthcare sector is now concerned on quality and accuracy of Point-of-Care (PoC) diagnostic test results. This apprehension is because of limited training or experience with these devices and those handling the same. Hands-on practice and ongoing education to remain updated with changes in technology are issues.
Pharmaceutical Technology
AUGUST 8, 2024
The contract will see bulk product for Jynneos replenished and additional services for dose storage.

Bio Pharma Dive
AUGUST 8, 2024
Company executives claimed “unprecedented demand” for the Duchenne gene therapy will soon cause a sales surge, countering a dip in quarterly sales and financial projections that raised concerns among investors.

Rethinking Clinical Trials
AUGUST 8, 2024
Speaking at the NIH Pragmatic Trials Collaboratory’s 2024 Annual Steering Committee Meeting in May, Commissioner of Food and Drugs Robert Califf and NIH Director Monica Bertagnolli discussed the intersection of pragmatic research and national health priorities. Califf and Bertagnolli spoke about the role the NIH Collaboratory can play in promoting scientifically rigorous, pragmatic clinical research that addresses the urgent need for better and faster implementation of research findings into pat

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
AUGUST 8, 2024
Analysts at Evercore ISI called pegcetacoplan’s data in two kidney conditions a “left-field hit” that compare favorably to results for a Novartis treatment.
Pharmaceutical Technology
AUGUST 8, 2024
The gene editing therapy will now be accessible to NHS England patients with beta-thalassemia, potentially replacing lifelong transfusions.

Bio Pharma Dive
AUGUST 8, 2024
Merck stopped a Phase 3 TIGIT study for futility. Elsewhere, shares in Cabaletta Bio sank on safety worries and Vertex secured Casgevy reimbursement in England.

Pharma Times
AUGUST 8, 2024
Lung cancer accounts for an estimated two million global diagnoses and 1.

Bio Pharma Dive
AUGUST 8, 2024
The two AI drug discovery firms, which have each lost most of their value since going public, believe their complementary skills can better speed the development of new medicines.
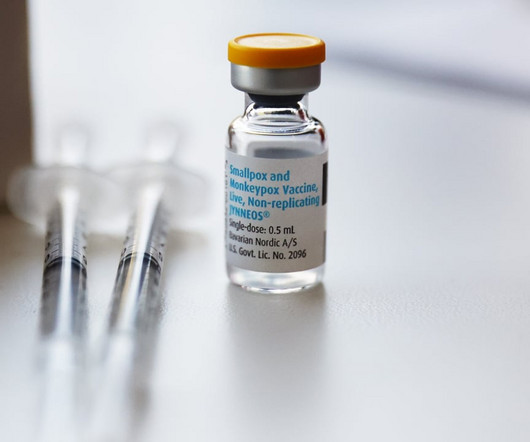
Fierce Pharma
AUGUST 8, 2024
Following the commercial launch of Bavarian Nordic’s smallpox/mpox vaccine Jynneos earlier this year, the Danish company has locked up yet another supply agreement with the U.S. government. | The Biomedical Advanced Research and Development Authority (BARDA) is handing Bavarian Nordic $156.8 million to “partly replenish” Jynneos vaccine stocks in response to the 2022 outbreak of mpox, the disease formerly known as monkeypox.

Pharmaceutical Technology
AUGUST 8, 2024
The US Food and Drug Administration (FDA) has approved Amneal Pharmaceuticals' CREXONT extended-release capsules for PD.

pharmaphorum
AUGUST 8, 2024
Recursion Pharma has agreed to merge with rival AI-powered drug discovery firm Exscientia in an all-stock deal valued at $688 million

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
AUGUST 8, 2024
Pharmacosmos will secure its second FDA-approved drug and first oncology therapy, G1 Therapeutics’ Costela as part of the merger.

Fierce Pharma
AUGUST 8, 2024
It was 2020 and Maryland biotech Novavax was thinking big. It had a plan to become a COVID vaccine powerhouse, with enough manufacturing capacity to supply the U.S. | As Novavax presented its quarterly earnings, finance chief Jim Kelly said the company was “actively exploring” the sale of its manufacturing facility in the Czech Republic. Novavax purchased the site in May of 2020 for $167 million with the intent of it producing more than 1 billion doses annually there.

Pharmaceutical Technology
AUGUST 8, 2024
GSK Singapore obtained the Singapore HSA approval for a new indication for Jemperli for advanced or recurrent endometrial cancer.

pharmaphorum
AUGUST 8, 2024
Roche is said to be considering a sale of cancer data specialist Flatiron Health, just a few years after it bought the company for $1.

BioPharma Reporter
AUGUST 8, 2024
With the FDAâs endorsement, Amgen and AstraZeneca are getting closer to adding chronic obstructive pulmonary disease (COPD) as a new indication to Tezspire.
pharmaphorum
AUGUST 8, 2024
Novartis gets FDA accelerated approval for Fabhalta in IgA nephropathy as it charts a course to blockbuster sales for the drug

Velocity Clinical Research
AUGUST 8, 2024
It’s been just over a year since the monoclonal antibody Lecanemab received traditional approval from the FDA as a treatment for Alzheimer’s disease. This marked a turning point in the disease’s treatment, a drug that interrupted progression for the first time rather than simply addressing the symptoms. It was this type of breakthrough that Robert Cupelo, MD hoped for when he joined Velocity’s Syracuse site as Principal Investigator in 2017.

pharmaphorum
AUGUST 8, 2024
Discover why pharmaceutical companies are trusted less than a waiter, but more than a pilot, and explore the role of Patient Advocacy Groups (PAGs) in shaping trust in the pharmaceutical industry.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Commerce
AUGUST 8, 2024
In this part of his Pharma Commerce video interview, Michael Rowe, Two Labs’ Senior Director of DSCSA/Serialization Compliance Services, discusses the state of affairs surrounding the Drug Supply Chain Security Act, along with opportunities for improvement.

pharmaphorum
AUGUST 8, 2024
Discover how your work habits and processes may be hindering your digital success in the pharmaceutical industry. Find out how you can invest in digital strategies for better outcomes.

Pharmaceutical Commerce
AUGUST 8, 2024
Business strategies and top news in the biotech / biopharma industry, including market access, supply chain distribution and more.
pharmaphorum
AUGUST 8, 2024
NICE backs Vertex's gene-editing therapy Casgevy as a treatment for beta thalassaemia, a few months after turning it down for sickle cell disease

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content