J&J antidepressant eases symptoms, improves sleep in key trial
Bio Pharma Dive
MAY 29, 2024
The company has put high expectations on the drug, an orexin-2 antagonist called seltorexant, as a cornerstone of its neuropsychiatry pipeline.

Bio Pharma Dive
MAY 29, 2024
The company has put high expectations on the drug, an orexin-2 antagonist called seltorexant, as a cornerstone of its neuropsychiatry pipeline.

Pharmaceutical Technology
MAY 29, 2024
Prothena plans to initiate a Phase I trial in an undisclosed indication by the end of 2024.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MAY 29, 2024
A partnership between cell therapy delivery specialist Portal Biotechnologies and precision robotics maker Multiply Labs could address manufacturing hurdles, the CEOs said.

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 29, 2024
A substance found in foods like pomegranates, strawberries, and walnuts restored the ability to detect and remove damaged cells in mice modeling Alzheimer’s disease, scientists report in a new paper. The same research team previously found a form of vitamin B3 called nicotinamide riboside (NR) helps remove damaged mitochondria from the brain.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Pharmaceutical Technology
MAY 29, 2024
Ensifentrine is a first-in-class dual phosphodiesterase 3/4 inhibitor to treat chronic obstructive pulmonary disease (COPD).

AuroBlog - Aurous Healthcare Clinical Trials blog
MAY 29, 2024
Home Editorial Services Interview Q&A Chronicle Specials Pharma Mart ePharmail Archives Join Pharma | Login Home > TopNews you can get e-magazine links on WhatsApp.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
MAY 29, 2024
The Series B funding from Two Sigma Ventures, RA Capital and others will help Gameto develop technology it says could replace hormonal injections and shorten the IVF process.

Pharmaceutical Technology
MAY 29, 2024
The US FDA has approved Amgen's Bkemv as an interchangeable biosimilar to Soliris for the treatment of specific rare diseases.

Bio Pharma Dive
MAY 29, 2024
An acquisition of EyeBio will hand Merck a treatment for diabetic macular edema and age-related macular degeneration that’s ready for pivotal testing.

Rethinking Clinical Trials
MAY 29, 2024
Dr. Shruti Gohil In this Friday’s PCT Grand Rounds, Shruti Gohil of the University of California, Irvine, will present “The INSPIRE Abdominal and Skin/Soft Tissue Infection Trials: Intelligent Stewardship Prompts to Improve Real-Time Empiric Antibiotic Selection for Patients.” The Grand Rounds session will be held on Friday, May 31, 2024, at 1:00 pm eastern.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharma Times
MAY 29, 2024
The proposals will help MedTech developers access NHS funding to fast-track products
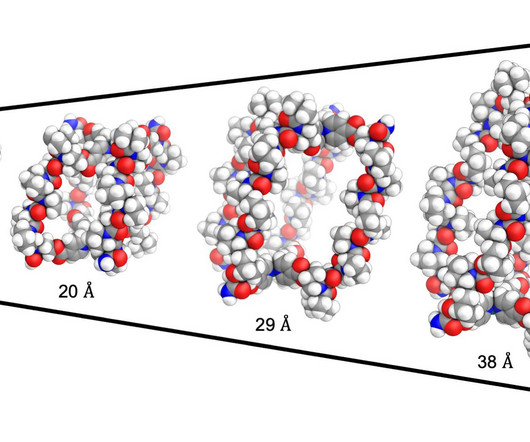
Pharmaceutical Technology
MAY 29, 2024
A team at King’s College London has developed a “drug delivery cage” that can be filled with chemotherapy drugs.

Pharma Times
MAY 29, 2024
Affecting more than 7 million people in the UK, cardiovascular diseases are conditions that affect the heart or circulation
Pharmaceutical Technology
MAY 29, 2024
Discover leading peptide manufacturers offering bespoke solutions and stringent quality assurance. Ensure your biotech projects succeed with our expert guide.

Antidote
MAY 29, 2024
Colorectal cancer, often shortened just to colon cancer, is the third most commonly diagnosed cancer in the United States aside from skin cancers. While there are treatments available, including radiation and chemotherapy, the most important aspect of successful treatment is early detection — which is why knowing the colon cancer screening guidelines is essential.

Pharmaceutical Technology
MAY 29, 2024
Vabysmo gained FDA approval in January 2022 in the wet age-related macular degeneration (wAMD) and diabetic macular oedema (DME) spaces.

pharmaphorum
MAY 29, 2024
More consolidation in the digital health sector as Akili agrees a $34m takeover by Virtual Therapeutics, returning to private hands.

Pharmaceutical Technology
MAY 29, 2024
The European Commission approved Bristol Myers Squibb’s Opdivo combination therapy for urothelial cancer based on Phase III data.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
MAY 29, 2024
In a new episode of the pharmaphorum podcast, Rahul Kakkar, CEO of gene editing company Tome, speaks with web editor Nicole Raleigh about programmable genomic integration (PGI) technology.

Pharmaceutical Technology
MAY 29, 2024
Merck KGaA has introduced the M-Trace Software & Mobile App to digitise sterility testing in microbial quality control.

pharmaphorum
MAY 29, 2024
MSD has confirmed a $3 billion takeover deal for ophthalmology biotech EyeBio and its drug candidate for diabetic macular oedema (DME) and neovascular age-related macular degeneration (AMD).

Pharmaceutical Technology
MAY 29, 2024
Cartography and Gilead Sciences partner to discover and develop therapies against new targets and target pairs in oncology.

pharmaphorum
MAY 29, 2024
AstraZeneca has reported the first clinical data with its oral PCSK9 inhibitor AZD0780, which it hopes could offer an alternative to current injectable drugs used to lower cholesterol.

Pharmaceutical Technology
MAY 29, 2024
Turn Bio has entered an agreement with HanAll Biopharma to develop therapies for age-related eye and ear ailments.

pharmaphorum
MAY 29, 2024
MSD is reported to be on the brink of agreeing a $1.

Pharmaceutical Commerce
MAY 29, 2024
The latest news for pharma industry insiders.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Fierce Pharma
MAY 29, 2024
Pfizer chalked up a key win in its legal battle surrounding the now-recalled smoking cessation drug Chantix after a New York judge narrowed the scope of the arguments in play. | The company still faces some claims in a class action lawsuit over the now-recalled smoking cessation med Chantix.
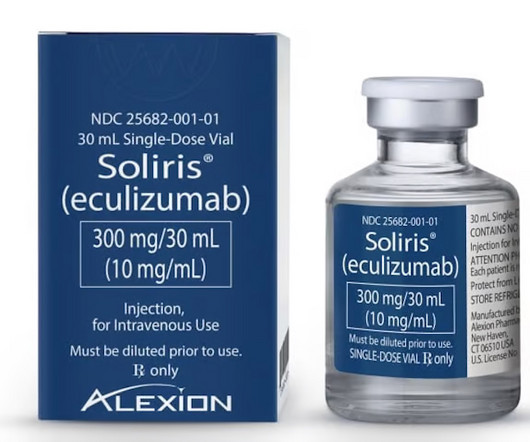
pharmaphorum
MAY 29, 2024
Amgen’s Bkemv is first FDA-approved biosimilar to AstraZeneca’s Soliris in the US, getting interchangeable status for rare diseases PNH and aHUS

Outsourcing Pharma
MAY 29, 2024
UK based Celadon Pharmaceuticals Plc, that specializes in the development, production, and sale of breakthrough cannabis-based medicines, announced that it has begun shipping to the US.

BioSpace
MAY 29, 2024
Tris Pharma on Wednesday secured the FDA’s green light for Onyda XR, the first liquid non-stimulant nighttime treatment for attention deficit hyperactivity disorder in pediatric patients.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content