Novo to acquire Dicerna for more than $3B amid RNA drug resurgence
Bio Pharma Dive
NOVEMBER 18, 2021
The acquisition is a rare deal for Novo Nordisk, which has been partnered with Dicerna since 2019.

Bio Pharma Dive
NOVEMBER 18, 2021
The acquisition is a rare deal for Novo Nordisk, which has been partnered with Dicerna since 2019.

World of DTC Marketing
NOVEMBER 5, 2021
SUMMARY: Wegovy is selling so well that it’s hard to get at pharmacies. It’s being positioned as an anti-obesity drug, but one study by Novo Nordisk has shown that people who stop taking Wegovy after a few months tend to regain much of their lost weight within a year. In addition, people who lost weight on Wegovy in clinical trials had nutritional counseling and had to stay on a strict diet.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Camargo
NOVEMBER 22, 2021
“There are only four kinds of people in the world: Those who have been caregivers, those who are currently caregivers, those who will be caregivers, and those who will need caregivers.”. ~ Roslyn Carter. Each November, National Family Caregiver Month recognizes and honors all those who have dedicated themselves to caring for ailing loved ones. It also helps raise awareness of the often-overwhelming issues they face, so that we can work together as a society to alleviate them.

Pharma Times
NOVEMBER 11, 2021
ChAdOxl biEBOV is being tested for safety and immunogenicity, and may protect against multiple species of the virus

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

NY Times
NOVEMBER 27, 2021
A new treatment using stem cells that produce insulin has surprised experts and given them hope for the 1.5 million Americans living with the disease.
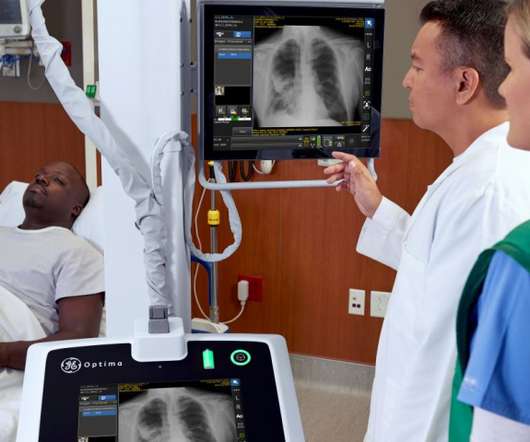
pharmaphorum
NOVEMBER 16, 2021
An artificial intelligence algorithm developed by GE Healthcare that helps with the placement of endotracheal tubes (ETTs) has been approved by the FDA. The new tool – part of GE’s Critical Care Suite 2.0 – helps bedside staff and radiologists assess patients before intubation – for example prior to ventilation in patients with critical COVID-19 – and make sure their ETTs are positioned correctly.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

World of DTC Marketing
NOVEMBER 24, 2021
SUMMARY: The U.S. needs to take substantial steps to address the high costs of cancer drugs. From 2009 to 2019, the median monthly treatment costs for new drugs at launch reached $11,755 in the U.S… Does the question become how much is a month(s) of other life worth? According to an analysis published by JAMA Oncology, prices for new drugs approved for use in the treatment of cancer in the United States more than doubled over the past decade.

Camargo
NOVEMBER 15, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. FDA Guidance Addresses Real-World Evidence Data Standards. The 21 st Century Cures Act generated a good deal of excitement and interest when it added a section called “ Utilizing Real World Evidence ” to the Food, Drug, and Cosmetic Act (Section 505F).

Pharma Times
NOVEMBER 25, 2021
A team of scientists and pharmaceutical collaborators have discovered a ‘bench to bedside’ drug design, which will hopefully improve the future treatment of Alzheimer’s disease.

JAMA Internal Medicine
NOVEMBER 7, 2021
This cross-sectional study of individuals from the French CONSTANCES cohort assesses whether the belief in having been infected with SARS-CoV-2, having serological test–confirmed COVID-19 infection, or both are associated with persistent physical symptoms often attributed to long COVID in the general adult population.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

pharmaphorum
NOVEMBER 28, 2021
An investigator-led trial of Jazz Pharma’s cannabis extract-based drug Sativex in glioblastoma – an aggressive form of brain cancer – will get underway in the UK next year. The three-year phase 2 trial will be carried out by researchers at Leeds University led by professor of clinical oncology and neuro-oncology Susan Short, and see if adding Sativex (nabiximols) to standard chemotherapy for recurrent glioblastoma can extend survival.

Bio Pharma Dive
NOVEMBER 26, 2021
Final trial data for molnupiravir were less convincing than what Merck previously reported and could alter how the FDA views the drug, which will be evaluated at a meeting Tuesday.
World of DTC Marketing
NOVEMBER 16, 2021
SUMMARY: Omnichannel marketing employs the simultaneous implementation of channels across personal, non-personal, and media and addresses the integrated needs of multiple stakeholders – consumers/patients, healthcare professionals, and payers. Sounds good but is that the answer? There is no doubt that healthcare is the middle of an evolution not seen in over 30 years.

Camargo
NOVEMBER 29, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. When developing biologics, sponsors must manage several scientific considerations specific to large molecule products, including biochemical characterization studies to confirm structural identity, biological activity studies to confirm potency, and mechanism of action maintenance.

Pharma Times
NOVEMBER 12, 2021
Researchers have said that next generation vaccines for COVID-19 should aim to induce a response against ‘replication proteins’

Scienmag
NOVEMBER 4, 2021
Since the first reported pangolin seizure in Nigeria in 2010, the country has seen an explosion in the black market for the world’s most trafficked mammal – becoming Africa’s hub for the criminal export of pangolin products to East Asia. Credit: Charles Emogor Since the first reported pangolin seizure in Nigeria in 2010, the country […].
pharmaphorum
NOVEMBER 12, 2021
Researchers in the US have developed an artificial intelligence-based tool that is able to predict COVID-19 symptoms and suggest which FDA-approved drugs might be used to treat patients. The MOATAI-VIR algorithm, developed by scientists at Emory University and Georgia Tech, was put through its paces in a study that showed it was able to predict 24 out of 26 clinical manifestations of COVID-19, including acute respiratory distress, blood clotting issues, cytokine storms, brain fog, and loss of sm

Bio Pharma Dive
NOVEMBER 19, 2021
The decision comes as at least 10 states have already begun opening up booster dose eligibility, with cases remaining at high levels across the country.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

World of DTC Marketing
NOVEMBER 22, 2021
SUMMARY: CVS announced its plans to begin closing its doors –about 900 locations across the country. They know the future isn’t about selling 20 kinds of shampoos and vitamins. In the words of the company’s mission , its goal is to “make high-quality health and pharmacy services safe, affordable and easy to access.” Growth doesn’t mean getting more extensive; it means getting better.

Pharma Mirror
NOVEMBER 26, 2021
In recent years, it has become more and more important to both consumers and investors that they support organisations which display a high level of social and environmental responsibility. This means that it’s no longer enough for businesses to manage their bottom lines and maintain profitability, they must also be conscious of the impact they have on the world around them.

Pharma Times
NOVEMBER 16, 2021
The data was obtained by a Freedom of Information request, according to The Independent

Deltaclinical
NOVEMBER 2, 2021
The sponsor of a study will assign one or multiple principle investigators (PI), usually a doctor. Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
NOVEMBER 16, 2021
Amazon’s push into the provision of remote healthcare services has been rewarded with a top-tier corporate client – the Hilton hotel chain. The deal – first reported by Reuters – involves the Amazon Care platform, which includes remote, online consultations with clinicians, as well as home visits in some areas. It provides primary and preventive care, ongoing support for chronic conditions, and referrals to secondary and tertiary care.

Bio Pharma Dive
NOVEMBER 30, 2021
While agency advisers raised concerns over molnupiravir's modest benefits and potential risks, a majority felt the antiviral drug is a needed option for COVID-19 patients at high risk of severe disease.

World of DTC Marketing
NOVEMBER 15, 2021
SUMMARY: Physicians are tired and overwhelmed with medical information on new drugs. They don’t have the time to sit in the office and search for more information, and they feel that pharma is giving them “too much” information. An exciting development presented itself. A client launched an Oncology drug based on extensive research with physicians, but the results were not that good during a post-launch evaluation.

Pharma Mirror
NOVEMBER 25, 2021
Terrassa (Barcelona) Telstar launches a new global service platform for retrofitting and maintenance of existing freeze-dryers, regardless of brand and model. Under Usifroid brand, this platform is specialized in providing a retrofitting and modernization service together with maintenance services and technical support to freeze-drying equipment for pharmaceutical production and research.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharma Times
NOVEMBER 30, 2021
Following its recent approval of an injectable HIV-1 treatment from the National institute for Health and Care Excellence (NICE), GlaxoSmithKline (GSK) has high hopes in developing a cure for the virus in the future.

Deltaclinical
NOVEMBER 2, 2021
The sponsor of a study will assign one or multiple principle investigators (PI), usually a doctor. Along with a team of nurses and other stakeholders, the PI will conduct the trial at his/her study site. However, the sponsor will remain the monitor of the trial. The next figure shows the important stakeholders in a clinical trial. Traditionally, clinical trials have never been designed with patient-centricity in mind.

pharmaphorum
NOVEMBER 3, 2021
A real-world study of digital therapeutic (DTx) for opioid use disorder has found that patient show used it had 46% fewer hospital stays than a control group, saving more than $2,700 over a nine-month period. The study of Pear Therapeutics’ reSET-O found that the total cost of hospital and clinician costs were $11,141 among 64 patients who were prescribed the DTx but didn’t use it, but fell to $8,733 among active users of the 12-week course.

Bio Pharma Dive
NOVEMBER 15, 2021
Luxturna, approved four years ago as the first gene therapy for an inherited disease in the U.S., is improving sight and quality of life for several of the patients who received it.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.
Let's personalize your content