Citryll gains Series B funds to develop NET-targeting antibody
Pharmaceutical Technology
DECEMBER 9, 2024
Citryll has secured an oversubscribed Series B funding round, raising 85m ($89.8m) to advance the clinical development of CIT-013.

Pharmaceutical Technology
DECEMBER 9, 2024
Citryll has secured an oversubscribed Series B funding round, raising 85m ($89.8m) to advance the clinical development of CIT-013.

Pharma Times
DECEMBER 9, 2024
Trial results lead to new treatment option for lung cancer
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
DECEMBER 10, 2024
Metabolic dysfunction-associated steatohepatitis (MASH), previously known as non-alcoholic steatohepatitis (NASH), is a growing health concern. In the US, MASH affects an estimated three to six percent of the population. This chronic liver disease results from fat accumulation that leads to liver cell damage and inflammation. It is often associated with obesity, type 2 diabetes and cardiovascular risks.

XTalks
DECEMBER 9, 2024
The US Food and Drug Administration (FDA) has released a new draft guidance titled Expedited Program for Serious Conditions Accelerated Approval of Drugs and Biologics Guidance for Industry refining the accelerated approval pathway for drugs and biologics targeting serious or life-threatening conditions. This guidance focuses on improved accountability, earlier confirmatory studies and greater clarity on novel endpoints.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
DECEMBER 9, 2024
Shares in WuXi AppTec, one of those implicated in the Chinese biotech blacklisting legislation, surged following the omission.

Rethinking Clinical Trials
DECEMBER 11, 2024
Dr. Gbenga Ogedegbe In this Friday’s PCT Grand Rounds, Gbenga Ogedegbe of the NYU Grossman School of Medicine will present “Home Blood Pressure Telemonitoring and Nurse Case Management in Black and Hispanic Patients With Stroke: A Randomized Clinical Trial.” The Grand Rounds session will be held on Friday, December 13, 2024, at 1:00 pm eastern.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Medical Xpress
DECEMBER 13, 2024
A new screening method that combines laser analysis with a type of AI is the first of its kind to identify patients in the earliest stage of breast cancer, a study suggests.

Pharmaceutical Technology
DECEMBER 12, 2024
In the letter, 77 Nobel Prize winners argue that appointing RFK as Secretary of HHS would put the US publics health in jeopardy.

Rethinking Clinical Trials
DECEMBER 12, 2024
In 2024, experts from the NIH Pragmatic Trials Collaboratory published the results of newly completed studies, shared insights from program leadership, and developed innovative methods in the design, conduct, and analysis of pragmatic clinical trials. Their work included perspectives from the Coordinating Center, best practices from the Core Working Groups , and results from the NIH Collaboratory Trials.

Pharma Times
DECEMBER 11, 2024
Successful phase 1 study highlights potential of roginolisib

Medical Xpress
DECEMBER 13, 2024
The devastating news of a cancer diagnosis understandably makes doctors and patients focus on the cancer itself. However, experts in cardio-oncology from the European Society of Cardiology (ESC) emphasize that heart and cardiovascular health must be included as early as possible in the patient's cancer treatment plan to ensure the best possible outcomes.

Pharmaceutical Technology
DECEMBER 12, 2024
Sanofi has announced that its two combination vaccine candidates have received fast track status from the US FDA.
XTalks
DECEMBER 10, 2024
Sanofis pivotal LUNA 3 Phase III study has delivered promising results for rilzabrutinib, marking an important advance for adults living with persistent or chronic immune thrombocytopenia (ITP). ITP is a rare autoimmune disorder that leads to dangerously low platelet counts. In this study, rilzabrutinib, an oral Brutons tyrosine kinase (BTK) inhibitor, demonstrated a durable platelet response in 23 percent of treated patients compared to none on placebo, indicating high statistical significance.

Pharma Times
DECEMBER 10, 2024
New cell therapy shows potential in treating advanced blood disorder

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Medical Xpress
DECEMBER 13, 2024
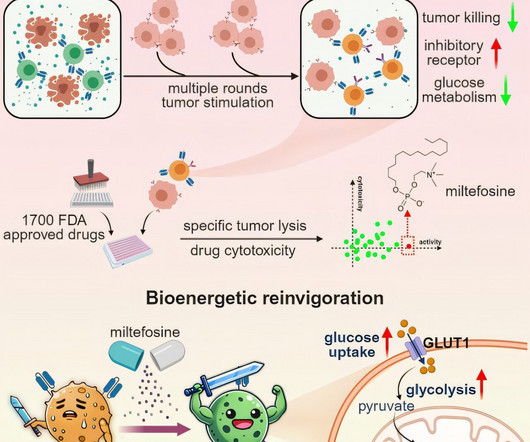
A research team led by Prof. Wang Haoyi from the Institute of Zoology (IOZ) of the Chinese Academy of Sciences has developed a chimeric antigen receptor T (CAR-T) cell exhaustion model and a functional screening platform for identifying compounds that can rejuvenate exhausted T cells.

Pharmaceutical Technology
DECEMBER 9, 2024
The US FDA has granted priority review to AstraZeneca's sBLA for Imfinzi (durvalumab) for muscle-invasive bladder cancer (MIBC) treatment.

XTalks
DECEMBER 11, 2024
AbbVie has announced encouraging topline results from its Phase III TEMPO-2 clinical trial, evaluating tavapadon as a monotherapy for early Parkinsons disease. The trial met its primary and key secondary endpoints, demonstrating significant improvements in motor and daily living functions. These findings underscore tavapadons potential as a first-in-class treatment option, addressing unmet needs with strong efficacy and manageable side effects.

Pharma Times
DECEMBER 10, 2024
New findings presented at international SCWD conference

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Medical Xpress
DECEMBER 13, 2024
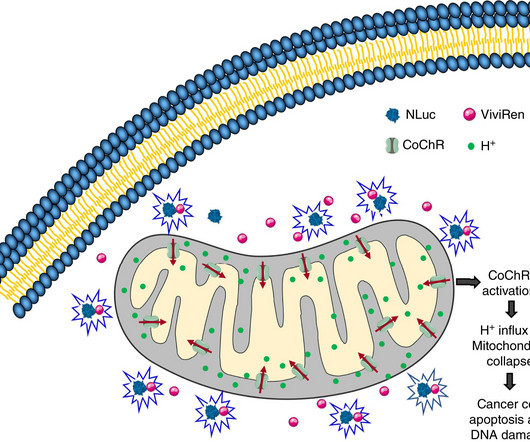
Researchers are shining a light on cancer cells' energy centersliterallyto damage these power sources and trigger widespread cancer cell death. In a new study, scientists combined strategies to deliver energy-disrupting gene therapy using nanoparticles manufactured to zero in only on cancer cells. Experiments showed the targeted therapy is effective at shrinking glioblastoma brain tumors and aggressive breast cancer tumors in mice.

Pharmaceutical Technology
DECEMBER 11, 2024
When a room of physicians at ASH 2024 was asked whether they had prescribed a gene therapy in a commercial context, only a handful said yes.

Drug Patent Watch
DECEMBER 10, 2024
Patents play a crucial role in protecting intellectual property and fostering innovation. However, the increasing complexity of patent systems has given rise to a phenomenon known as “patent thickets.” These dense webs of overlapping patent rights can significantly impact innovation, market entry, and competition across various industries and countries.

Pharma Times
DECEMBER 12, 2024
Trial explores potential of therapy in treating multiple cancer types

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Medical Xpress
DECEMBER 13, 2024
Researchers at the University of Bergen have used advanced stem cell technology to develop mini-brains, also called brain organoids, that can mimic disease processes caused by mitochondrial failure. This could open new avenues for treating serious brain diseases such as epilepsy.
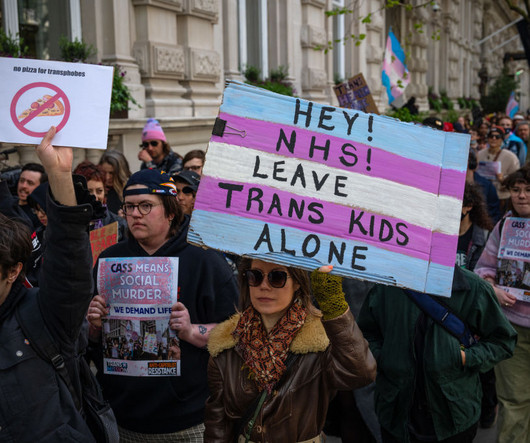
Pharmaceutical Technology
DECEMBER 12, 2024
Puberty blockers for people aged under 18 with gender dysphoria will be indefinitely banned by the UK Government.
Drug Patent Watch
DECEMBER 12, 2024
Identifying branded drugs with a low likelihood of generic entry has become a crucial strategy for companies looking to expand their product portfolio through in-licensing. This approach not only helps maintain market exclusivity but also ensures a steady revenue stream for pharmaceutical companies. In this comprehensive guide, we’ll explore the intricacies of identifying such drugs and leveraging them for successful in-licensing opportunities.

Pharma Times
DECEMBER 9, 2024
promising pre-clinical data on preventing cytokine release syndrome

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.
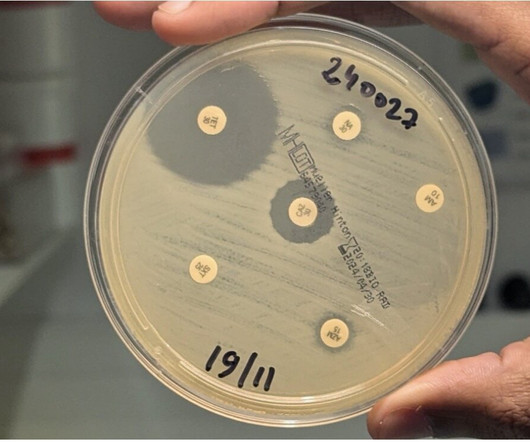
Medical Xpress
DECEMBER 13, 2024
Scientists from the National Reference Center for Vibrios and Cholera at the Institut Pasteur, in collaboration with the Center hospitalier de Mayotte, have revealed the spread of a highly drug-resistant cholera strain. The study was published on December 11, 2024 in the New England Journal of Medicine.

Pharmaceutical Technology
DECEMBER 11, 2024
Er-Kim has extended its exclusive agreement with Ascendis Pharma for endocrinology treatments across multiple countries in Eurasia.

Drug Patent Watch
DECEMBER 9, 2024
In the vast realm of pharmaceutical research and development, there’s a fascinating intersection between ancient wisdom and modern science. This intersection is where pharmacognosy meets drug patents, creating a unique landscape that shapes the future of medicine. But what exactly is pharmacognosy, and how does it relate to the complex world of drug patents?

Pharma Times
DECEMBER 12, 2024
Promising trial results support marketing authorisation application
Let's personalize your content