Clinical Research Trends & Insights for 2024 V4
WCG Clinical
DECEMBER 28, 2023
This content is password protected. To view it please enter your password below: Password: The post Clinical Research Trends & Insights for 2024 V4 appeared first on WCG.

WCG Clinical
DECEMBER 28, 2023
This content is password protected. To view it please enter your password below: Password: The post Clinical Research Trends & Insights for 2024 V4 appeared first on WCG.

ProRelix Research
DECEMBER 28, 2023
Considering the crucial role that the information generated from clinical trials play in the approval of new drugs, biological, and medical devices, it is only logical that the data garnered […] The post Clinical Data Standardization in Clinical Trials: FDA Compliance in Clinical Data Management appeared first on ProRelix Research.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Roots Analysis
DECEMBER 29, 2023
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug’s safety and effectiveness towards a specific target indication.

Antidote
DECEMBER 27, 2023
This interview features Dr. Aaron Kowalski from JDRF, who discusses diabetes clinical trials, upcoming research, and more.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

WCG Clinical
DECEMBER 27, 2023
This content is password protected. To view it please enter your password below: Password: The post Diversity, Equity, and Inclusion in Rare Disease Clinical Trials: Current Opportunities and Outcomes appeared first on WCG.

pharmaphorum
DECEMBER 27, 2023
Patient organisations play a crucial role in the rapidly evolving landscape of healthcare, life sciences, and research. Learn about their role in advocacy, research, and navigating regulatory processes in this informative article.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

XTalks
DECEMBER 27, 2023
PatientRightsAdvocate.org (PRA), a leading non-profit organization championing the cause of healthcare price transparency in America, launched Hospital Price Files Finder , a freely available dashboard tool containing pricing files for 6,000 hospitals nationwide. It is a searchable tool containing all available hospital pricing files in spreadsheets that are human and machine-readable, converted from the original format as necessary.

Pharmaceutical Technology
DECEMBER 29, 2023
FDA warns that levetiracetam and clobazam can cause DRESS, a rare hypersensitivity reaction which can be life threatening.

Fierce Pharma
DECEMBER 27, 2023
Amgen’s request to gain full approval of Lumakras in non-small cell lung cancer has been rejected by the FDA. | Amgen’s request to gain full approval of Lumakras in non-small cell lung cancer has been rejected by the FDA. The California biopharma will now have to conduct an additional confirmatory trial to gain the coveted FDA nod.

pharmaphorum
DECEMBER 27, 2023
Bristol-Myers Squibb makes a foray into the radiopharmaceutical category with a $4.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
XTalks
DECEMBER 29, 2023
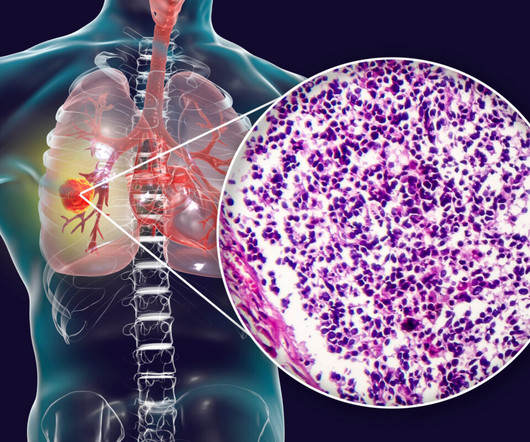
Amgen recently reported that the US Food and Drug Administration (FDA) has given priority review status to its drug candidate, tarlatamab, intended for the treatment of small-cell lung cancer (SCLC). If approved, tarlatamab could bring about significant improvements compared to existing options or potentially offer a treatment alternative in cases where no adequate therapy currently exists. “The FDA’s Priority Review designation for this application underscores the urgency to provide

Pharmaceutical Technology
DECEMBER 28, 2023
The MHRA gave Bristol Myers Squibb’s Opdualag marketing authorisation for advanced melanoma as part of Project Orbis.

Fierce Pharma
DECEMBER 27, 2023
After a year plagued by recalls, Pfizer’s sterile injectables unit Hospira seems no closer to righting the ship over its glass-contamination woes. | After a year plagued by recalls, Pfizer’s sterile injectables unit Hospira seems no closer to righting the ship over its glass-contamination woes. Shortly before the holiday, Hospira announced two separate recalls over the potential presence of glass particulates in vials and syringes of certain hospital meds.
pharmaphorum
DECEMBER 29, 2023
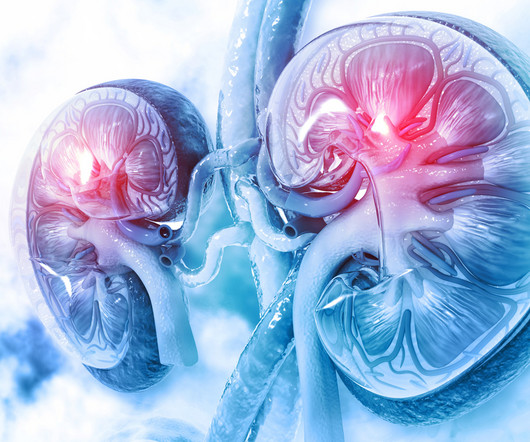
Navigating transplant networks: Ensuring equitable access and improving outcomes for highly sensitised kidney transplant patients Mike.

XTalks
DECEMBER 26, 2023
Karuna Therapeutics, a biopharmaceutical company dedicated to uncovering, advancing and providing groundbreaking medicines for individuals facing psychiatric and neurological conditions, has recently disclosed encouraging outcomes from the Phase III EMERGENT-2 trial of KarXT (xanomeline-trospium) in adults diagnosed with schizophrenia. “New treatments and novel mechanisms are urgently needed for people with schizophrenia because many don’t respond to their therapy and others only have a partial

Pharmaceutical Technology
DECEMBER 27, 2023
The deal is worth approximately $1.2bn in combined upfront and potential contingent value payments and is expected to close in Q1 2024.

Fierce Pharma
DECEMBER 29, 2023
Armed with a new victory over Viatris in a patent dispute, Regeneron can knock off one contender in the Eylea biosimilar race. | A West Virginia judge found that Viatris' proposed biosimilar stepped on one of the three patents that Regeneron sued over. Shortly after, the company expanded its suit against another biosimilar contender, setting the stage for Regeneron's defense in Eylea's last few months of exclusivity.

pharmaphorum
DECEMBER 29, 2023
Eisai and Oita University have developed a machine learning-powered wearable that can predict whether someone is at risk of developing Alzheimer’s disease

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

JAMA Internal Medicine
DECEMBER 25, 2023
This randomized clinical trial examines whether an intensive food-as-medicine program can improve glycemic control and ,1rengagement with preventive health care compared with usual care among adults with diabetes and food insecurity.

Pharmaceutical Technology
DECEMBER 28, 2023
J&J has acquired a global license for South Korean LegoChem’s ADC for $100m upfront along with milestone-based payments and tiered royalties.

Drug Patent Watch
DECEMBER 29, 2023
This chart shows the pharmaceutical companies with the most gel dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most gel dosed drugs… The post Which pharmaceutical companies have the most gel dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
DECEMBER 26, 2023
AstraZeneca agrees a $1.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Imperical Blog
DECEMBER 27, 2023
The Imperial blog team took a look at our 2023 clinical trial blogs, and we’re shining the spotlight on our top six picks. So here they are, in no particular order: six clinical trial blogs worth reading (and re-reading). 1. Supply Chain: Master these key… The post 6 Top Picks: Clinical Trial Blogs Not to Miss appeared first on Imperial Clinical Research Services Blog.

Pharmaceutical Technology
DECEMBER 29, 2023
Enveric Biosciences has selected EB-003 as its lead drug candidate from its EVM301 series for difficult-to-treat mental health disorders.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for SAXENDA Saxenda is a drug marketed by Novo and is included in one NDA. It is available from one supplier. There are twenty-one patents protecting… The post New patent expiration for NOVO drug SAXENDA appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
DECEMBER 23, 2023
BMS has reached a $14bn agreement to buy Karuna and its KarXT schizophrenia candidate, due for an FDA verdict next September

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmaceutical Commerce
DECEMBER 29, 2023
Popular works in the category explore 340B guidelines, ‘smart pharma’ capabilities, and commercialization trends.

Pharmaceutical Technology
DECEMBER 26, 2023
Bristol Myers Squibb (BMS) has signed a definitive agreement for the acquisition of Karuna Therapeutics for $14bn in cash.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for CHLORAPREP+WITH+TINT Chloraprep With Tint is a drug marketed by Becton Dickinson Co and is included in one NDA. It is available from two suppliers. There… The post New patent expiration for Becton Dickinson drug CHLORAPREP WITH TINT appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
DECEMBER 28, 2023
Cytokinetics rises on report that its aficamten drug improved symptoms in people with rare disease obstructive hypertrophic cardiomyopathy
Let's personalize your content