7 best practices for assessing eCOA technology solutions
pharmaphorum
JULY 17, 2023
7 best practices for assessing eCOA technology solutions Mike.

pharmaphorum
JULY 17, 2023
7 best practices for assessing eCOA technology solutions Mike.

BioSpace
JULY 16, 2023
Bringing new drugs to the market costs billions of dollars. It could not be done without investments by both the NIH and biopharma companies.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
JULY 18, 2023
The unusual research alliance involves the startup creator’s Pioneering Medicines initiative, which previously struck pacts with Novo Nordisk and the Cystic Fibrosis Foundation.

Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
JULY 17, 2023
New data presented Monday at a research conference support the case for Lilly’s donanemab, which works in a similar way as the two Alzheimer’s medicines recently approved in the U.S.

Bio Pharma Dive
JULY 17, 2023
For $500 million, Novartis will acquire DTx Pharma and its preclinical neurological disease drugs, marking the Swiss company’s latest investment in gene-silencing medicines.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
JULY 17, 2023
An organization of retinal specialists flagged six cases of severe eye inflammation from commercial use of Syfovre. Apellis says the drug’s safety profile is consistent with testing.

Pharmaceutical Technology
JULY 17, 2023
The US Federal Court overturned a previous ruling in the patent infringement case against Rockefeller University.

Pharmaceutical Technology
JULY 19, 2023
Advent Therapeutics has received a grant worth $3m from the US NIH for expediting the development of bronchopulmonary dysplasia therapy.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 20, 2023
Ketamine might be better known as a recreational drug or anesthetic. But there’s growing evidence for its use for people with hard-to-treat depression. An Australasian study out today [July 14] showed some positive results for people with treatment-resistant depression when they had ketamine injections.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
JULY 21, 2023
The offering is a financial lifeline for Turnstone and the fourth for the sector this month, the first time that’s happened in nearly a year and a half, according to BioPharma Dive data.

Rethinking Clinical Trials
JULY 19, 2023
From left: Dr. Christine Goertz and Dr. Adam Goode of the IMPACt-LBP Demonstration Project In this Friday’s PCT Grand Rounds, Dr. Christine Goertz and Dr. Adam Goode of Duke University will present “Implementing New Care Pathways for Low Back Pain in Academic Healthcare Systems: Early Lessons From IMPACt-LBP.” The Grand Rounds session will be held on Friday, July 21, 2023, at 1:00 pm eastern.

Pharmaceutical Technology
JULY 19, 2023
Twist Bioscience and CRUK innovation arm Cancer Research Horizons have signed an agreement for licensing a library of libraries.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 18, 2023
The International Agency for Research on Cancer (IARC), which is the specialized cancer agency of the World Health Organization, has declared aspartame may be a possible carcinogenic hazard to humans.

Bio Pharma Dive
JULY 21, 2023
The Swiss drugmaker now joins a lengthy list of biotechs that, amid a drawn-out market downturn, have turned to job cuts and research re-evaluations to save money.

Rethinking Clinical Trials
JULY 18, 2023
Dr. Lynn DeBar and Dr. Natalia Morone In an interview at the annual NIH Pragmatic Trials Collaboratory Steering Committee meeting, Dr. Lynn DeBar and Dr. Natalia Morone had a conversation about sharing trial results with participant partners. Both also participated in a discussion session about the challenges and value of results dissemination. DeBar is a principal investigator of the BackInAction and PPACT Demonstration Projects, and Morone is the principal investigator of the OPTIMUM Demonstra

Pharmaceutical Technology
JULY 19, 2023
B. pseudomallei is resistant to many antibiotics and, without treatment, can be lethal to those infected.
AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 17, 2023
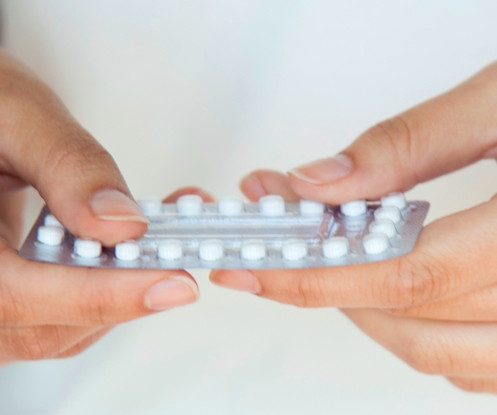
On July 13, 2023, the U.S. Food and Drug Administration approved a drugmaker’s application for the first daily over-the-counter birth control pill for people seeking to prevent pregnancy.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
JULY 19, 2023
The agency veteran, who led the Office of Tissues and Advanced Therapies during a boom in gene and cell therapy research, retired in March.

Rethinking Clinical Trials
JULY 17, 2023
From left: Dr. Stephanie Ibemere and Dr. Kaitlyn McLeod The NIH Pragmatic Trials Collaboratory is welcoming its first class of fellows in a new program for early-career investigators from underrepresented or minoritized groups with a scholarly interest in pragmatic clinical trials. “We are very excited to welcome the 2 inaugural fellows to the NIH Pragmatic Trials Collaboratory Fellowship Program and give them the education and tools that they need to launch their careers as pragmatic clin

Pharmaceutical Technology
JULY 21, 2023
Hutchmed has secured breakthrough therapy designation from China’s NMPA for fruquintinib and sintilimab combination to treat EMC.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 16, 2023
Alzheimer’s disease is often thought of as a condition that only affects the elderly. But around 3.9 million people worldwide aged 30-64 live with young-onset Alzheimer’s disease – a form of dementia in which symptoms appear before the age of 65.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Bio Pharma Dive
JULY 17, 2023
Meet the pharma marketer of the future, primed for personalization and powered by technology.
Pharma Times
JULY 17, 2023
OCTP develops cannabinoid medicines and is looking at potential treatment of cancer malignancies - News - PharmaTimes

Pharmaceutical Technology
JULY 17, 2023
The move follows a positive decision made by the EMA earlier this year, broadening the use of Veklury to the high-risk patient group.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 18, 2023
Artificial intelligence (AI) is set to make tectonic shift in healthcare with accuracy in diagnostics and predictability of disease. On the occasion of the Artificial Intelligence Appreciation Day observed annually on July 16, 2023, with the theme as ‘trust, ethics, innovation and public good’, industry experts see a paradigm shift on the healthcare landscape.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Bio Pharma Dive
JULY 18, 2023
The startup, a developer of gene therapies for brain diseases like Rett Syndrome, will bypass an IPO by merging with struggling cancer drugmaker Neoleukin.

Pharma Times
JULY 18, 2023
Company’s phase 3 research showed the importance of running biomarker-powered clinical trials - News - PharmaTimes

Pharmaceutical Technology
JULY 21, 2023
While artificial intelligence (AI) in medical devices has regulatory boundaries, its use in a medicine’s life cycle is less clear.

AuroBlog - Aurous Healthcare Clinical Trials blog
JULY 20, 2023
The Directorate General of Foreign Trade (DGFT) has notified a curriculum for the industry-led skilling and mentorship initiative towards equipping Status Holders as per Para 1.30 of Foreign Trade Policy (FTP) – 2023. This will further improve the pharma trade ecosystem by enhancing the available skilling opportunities.
Let's personalize your content