Pfizer cleared to restart hemophilia gene therapy trial
Bio Pharma Dive
MAY 3, 2022
While a voluntary pause in dosing new patients will remain in place, the FDA's decision puts the study back on track to deliver data in 2023.

Bio Pharma Dive
MAY 3, 2022
While a voluntary pause in dosing new patients will remain in place, the FDA's decision puts the study back on track to deliver data in 2023.

World of DTC Marketing
MAY 4, 2022
A 2015 Commonwealth Fund brie f showed that — before the major provisions of the Affordable Care Act were introduced — the United States had worse outcomes and spent more on health care, primarily because of greater use of medical technology and higher prices, compared to other high-income countries. The United States ranks last overall, despite spending far more of its gross domestic product on health care.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
MAY 4, 2022
Testing drug compounds on a chip designed to mimic human organs sounds closer to science fiction than reality, yet the technology already exists and is already being put to use. Ben Hargreaves discovers how the technology could provide more accurate safety predictions and even discover new treatments. The limits of animal models in drug discovery are well known.
BioPharma Reporter
MAY 5, 2022
Oramed Pharmaceuticals announced this week that it has enrolled 100% of the patients in the worldâs first Phase 3 study of oral insulin under FDA approved protocols.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Bio Pharma Dive
MAY 3, 2022
Michel Vounatsos, Biogen's CEO since 2017, will be replaced as the company "substantially" eliminates the commercial workforce around Aduhelm, which has generated paltry sales in the face of resistance from insurers and doctors.

World of DTC Marketing
MAY 3, 2022
Biogen announced that CEO Michel Vounatsos is being replaced as the big biotech undergoes a restructuring of the pipeline. It’s long overdue but kind of like putting smoke detectors in a house that has burnt down. From the beginning, Aduhelm was doomed to fail. There was outrageous pricing along with data that, at best, was highly questionable.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharma Times
MAY 6, 2022
WHO have stated that Veklury recommendation is being updated following compelling new evidence of hospital reductions

Bio Pharma Dive
MAY 2, 2022
The regulator cited concerns around single-country trials in turning back Hutchmed's pancreatic cancer treatment, while manufacturing issues held up Junshi and Coherus' throat cancer medicine.

Drug Patent Watch
MAY 2, 2022
DrugPatentWatch has been named to Genetic Engineering News‘ Best of the Web list. The review by Genetic Engineering News profile highlighted the “new training modules designed to help you find…. The post DrugPatentWatch named “Best of the Web” by Genetic Engineering News appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
MAY 4, 2022
Cognitive problems affecting memory and attention can be substantial and long-lasting in patients who suffer severe COVID-19 infections, according to a study by researchers in the UK. The study used computerised cognitive assessments to follow-up 46 people who were hospitalised with COVID-19 at the Addenbrooke’s Hospital in Cambridge over a three-month period in 2020, comparing the results with 460 matched control subjects.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharma Times
MAY 5, 2022
DELIVER represents the largest ever trial to address heart failure with preserved ejection fraction

Bio Pharma Dive
MAY 6, 2022
The agency is limiting use to adults who either can't or won't take another authorized vaccine after collecting more data on a rare and unusual clotting syndrome that has weighed on the shot's uptake.

XTalks
MAY 3, 2022
Whipnotic is putting a new twist on whipped cream, using its patented technology to dispense a creamy whipped topping with flavor-infused swirls and all-natural colors. The New York-based startup delivers an entirely reimagined multi-sensory whipped cream experience, revolutionizing the relatively unchanged whipped cream market. . Whipnotic is the first swirled whipped cream that uses its patented technology to dispense an all-natural color and infused swirl with the press of the nozzle.

pharmaphorum
MAY 3, 2022
Alain Labrique, PhD, professor and associate chair of research at Johns Hopkins Bloomberg School of Public Health (JHSPH) and chair of the World Health Organization (WHO) digital health guidelines development group discusses with us the ongoing research study, National Pandemic Pulse, he and his colleagues are performing in the US. Using comprehensive, repeat surveys deployed across the US population, Labrique and fellow researchers at JHSPH are measuring disparities and inequities due to COVID

Pharma Times
MAY 4, 2022
Platform will enable patients to monitor and share updates of their post-surgical wound healing

Bio Pharma Dive
MAY 2, 2022
The regulator determined Vertex had "insufficient information" to test higher doses of the therapy, which showed promise in the first two patients treated.

BioPharma Reporter
MAY 3, 2022
Biogen will âsubstantially eliminateâ commercial infrastructure for Alzheimerâs drug Aduhelm and take additional cost-reduction measures, the company announced as it released its Q1 2022 results this morning.
XTalks
MAY 6, 2022
In this interview, Xtalks spoke with experts from eClinical Solutions , Katrina Rice, Chief Delivery Officer, Data Services; and Diane Lacroix, Vice President, Clinical Data Management, about clinical data management for modern day digital clinical trials. They discussed current challenges and solutions that life science companies and clinical development organizations have with the management of clinical data.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharma Times
MAY 6, 2022
Norfolk and Norwich University Hospitals Foundation Trust opens centre to increase survival rate among pregnant women managing other conditions

Bio Pharma Dive
MAY 2, 2022
The partners increased the size and length of a large, ongoing study in transthyretin amyloidosis cardiomyopathy, a decision that has implications Alnylam Pharmaceuticals, which is testing two of its drugs against the disease.

BioPharma Reporter
MAY 4, 2022
An expert from Pharmatech Associates offers a glimpse into the evolution of CM and previews a lively discussion during the upcoming CPhI North America event.

XTalks
MAY 5, 2022
The Paleo Diet LLC, the founding organization behind the corresponding lifestyle movement, has launched a new Paleo certification program. Based on the science from the researchers who established the framework of modern Paleolithic nutrition, the program aims to provide guidance for the food industry by codifying Paleo Diet standards and making them available to manufacturers, retailers and other partners.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharma Times
MAY 3, 2022
Byondis and Medac have entered into a license and collaboration agreement for form of targeted chemotherapy

Bio Pharma Dive
MAY 4, 2022
Agency statisticians took a different view of the Phase 3 results the biotech was relying on to support approval of a new antibiotic, an announcement that triggered the sector's latest restructuring.

BioPharma Reporter
MAY 5, 2022
ExcellGene SA, a Swiss biologics focused CDMO, has established a collaboration agreement with German biological cell instrument developer, Cytena.
XTalks
MAY 4, 2022
Bristol Myers Squibb (BMS) was granted US Food and Drug Administration (FDA) approval for its highly awaited cardiac drug Camzyos (mavacamten) for the treatment of symptomatic obstructive hypertrophic cardiomyopathy (HCM). The FDA approved 2.5 mg, 5 mg, 10 mg and 15 mg capsules of Camzyos for adults with symptomatic New York Heart Association (NYHA) class II-III obstructive HCM to improve functional capacity and symptoms.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharma Times
MAY 3, 2022
The EC’s decision on the insomnia treatment was supported by phase 3 trial results

Bio Pharma Dive
MAY 4, 2022
Though executives claimed that inking deals and derisking research programs are priorities, analysts questioned whether any meaningful strategic changes will occur in the near term.
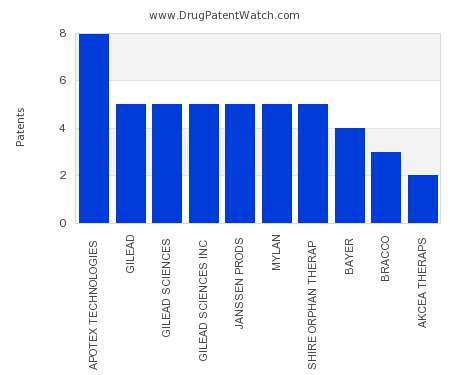
Drug Patent Watch
MAY 6, 2022
This chart shows the pharmaceutical companies with the most patents in Ireland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Ireland? appeared first on DrugPatentWatch - Make Better Decisions.
XTalks
MAY 5, 2022
Supernus Pharmaceuticals’ Qelbree was approved by the FDA on Friday. Qelbree is the newest nonstimulant ADHD drug for adults after two decades. Attention deficit hyperactivity disorder (ADHD) is one of the most established psychiatric disorders found in children and can be diagnosed at 3 years of age. The common symptoms of ADHD include lack of attention, hyperactivity and increased impulsivity.
Let's personalize your content