The state of the clinical trial supply chain: what you need to know
Pharmaceutical Technology
DECEMBER 6, 2023
The clinical trial market is in a state of flux; to navigate it, supply chain optimisation will be essential.

Pharmaceutical Technology
DECEMBER 6, 2023
The clinical trial market is in a state of flux; to navigate it, supply chain optimisation will be essential.

Worldwide Clinical Trials
DECEMBER 7, 2023
Amidst a shifting clinical landscape characterized by increasingly complex trial designs and growing patient subpopulations, many contract research organizations (CROs) have adopted a “one-stop-shop” strategic approach. As a result, various CROs have undergone significant consolidations and acquisitions of specialized capabilities to address the escalating complexity in clinical trials.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
DECEMBER 4, 2023
With clinical trials becoming more complex, finding ways to streamline and automate laboratory processes is vital when analyzing bioanalytical samples.

Rethinking Clinical Trials
DECEMBER 6, 2023
In this Friday's PCT Grand Rounds, Ruth Engelberg, Erin Kross, and Robert Lee of the University of Washington will present "A Pragmatic Randomized Trial of the Jumpstart Intervention to Promote Communication About Goals of Care for Hospitalized Patients With Serious Illness." The Grand Rounds session will be held on Friday, December 8, 2023, at 1:00 pm eastern.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Worldwide Clinical Trials
DECEMBER 5, 2023
Author: Lona Sheeran, SVP, Clinical Operations, Early Phase At this year’s Clinical Trials Nexus, I had the privilege of representing Worldwide Clinical Trials as the sole CRO on a panel discussion: “Reversing the Conversation: What the Clinical Trial Industry Really Wants from its Service Providers.” Among the panel participants included representatives from Teva, Blue Rock Therapeutics, and BMS.

Bio Pharma Dive
DECEMBER 4, 2023
Accurate and timely processing of data is imperative to create robust analytical datasets that can be used in the RWE setting.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
BioSpace
DECEMBER 3, 2023
From the skin to the lungs to the central nervous system, biotech companies are making progress toward delivering RNA therapeutics to multiple targets throughout the body. But challenges remain.

BioSpace
DECEMBER 4, 2023
The gene editing company is dropping two programs and favoring its next-generation assets CTX112 and CTX131, which it will continue to develop in oncology but will also test in autoimmune diseases.

Bio Pharma Dive
DECEMBER 8, 2023
In addition to clearing Vertex Pharmaceuticals and CRISPR Therapeutics’ Casgevy, the FDA also granted an early OK to Bluebird bio’s sickle cell treatment Lyfgenia.
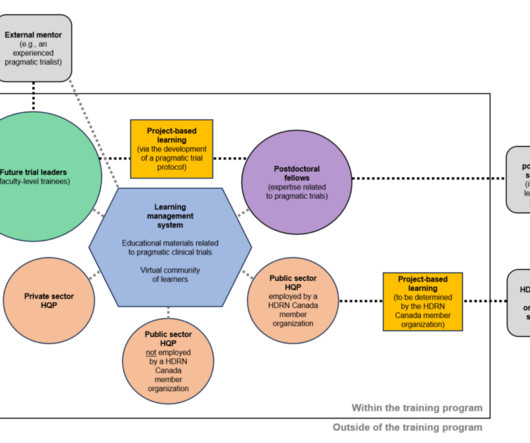
Rethinking Clinical Trials
DECEMBER 5, 2023
Health Data Research Network (HDRN) Canada is now accepting applications for its Pragmatic Trials Training Program. This 2-year, virtual, pan-Canadian program will provide training to advanced learners across 3 streams: (1) future trial leaders (faculty-level trainees), (2) postdoctoral fellows, and (3) highly qualified personnel employed in the public and private sectors.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
DECEMBER 6, 2023
Seattle Children's launched BrainChild Bio to focus on expediting the development of CAR T-cell therapies in central nervous system tumours.

Pharma Times
DECEMBER 4, 2023
In England, an estimated 4,500 people are living with undiagnosed HIV - News - PharmaTimes

Bio Pharma Dive
DECEMBER 7, 2023
The funding is indicative of investor interest in an area of drug research that involves at least a dozen startups and multiple publicly traded companies.

FDA Law Blog
DECEMBER 4, 2023
By Larry K. Houck — Separate decisions by federal district courts in Texas and Puerto Rico in the past two months provide cautionary tales for every pharmacy and wholesale distributor dispensing or distributing controlled substances. On October 10th, based on ability to pay, the U.S. District Court for the Western District of Texas imposed a $275,000 civil penalty on Zarzamora Healthcare LLC, in San Antonio, and its pharmacist-owner.
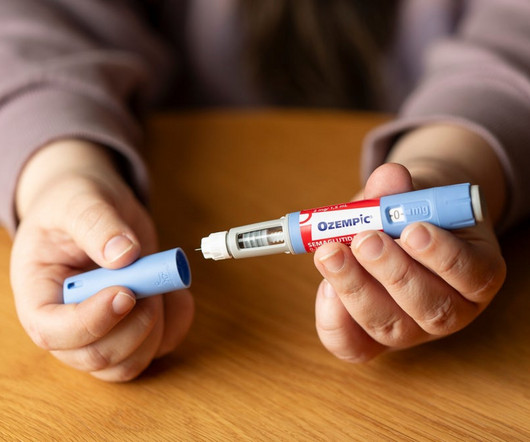
Pharmaceutical Technology
DECEMBER 5, 2023
Professor Timothy Mackey talks about the recent reports of fraudulent Ozempic pens and the challenges in chasing drug counterfeiters.

Pharma Times
DECEMBER 7, 2023
The disease is one of the leading causes of cancer deaths in the UK - News - PharmaTimes

Bio Pharma Dive
DECEMBER 6, 2023
The deal hands AbbVie a portfolio of psychiatric medicines that originated within Pfizer, among them a closely watched schizophrenia treatment that’s in late-stage testing.

Pharma Mirror
DECEMBER 6, 2023
Oral health is a critical component of overall well-being, and growing research suggests that the health of your mouth is closely connected to your body’s overall health. The mouth serves as a gateway to the body, and any issues or infections that begin in the oral cavity can impact other systems and organs. We will explore the intricate relationship between oral health and overall body health, shedding light on how taking care of your teeth and gums can benefit your well-being.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
DECEMBER 7, 2023
The FDA has granted fast track designation to Solid Biosciences’s gene therapy SGT-003 for the treatment of DMD.

Pharma Times
DECEMBER 8, 2023
One-third of patients who received Pfizer’s COVID-19 vaccine had unintended immune responses - News - PharmaTimes

Bio Pharma Dive
DECEMBER 8, 2023
Casgevy, the first CRISPR therapy approved by the FDA, will cost $2.2 million, while a competing genetic medicine also cleared Friday is priced at $3.1 million.

Fierce Pharma
DECEMBER 6, 2023
With an FDA approval in PNH, Novartis has gained its first nod for iptacopan, dubbed two months ago “a pipeline in pill," by analysts at ODDO BHF.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
DECEMBER 7, 2023
Japan has granted approval for the sBLA of Bristol-Myers Squibb (BMS) for Abecma to treat relapsed or refractory multiple myeloma (RRMM).

Pharma Times
DECEMBER 5, 2023
The platform safely and effectively replicates the electric signalling process compounds - News - PharmaTimes

Bio Pharma Dive
DECEMBER 6, 2023
The pharma is developing Fabhalta, now cleared for paroxysmal nocturnal hemoglobinuria, for several other rare, complement-driven diseases.

Fierce Pharma
DECEMBER 8, 2023
Alongside a historic approval for the first therapy utilizing the Nobel Prize-winning CRISPR/Cas9 gene-editing technology, the FDA has cleared a rival gene replacement therapy, also for sickle cell | Alongside a historic approval for the first therapy utilizing the Nobel Prize-winning CRISPR/Cas9 gene-editing technology, the FDA has cleared a rival gene replacement therapy, also for sickle cell disease (SCD).

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmaceutical Technology
DECEMBER 8, 2023
The newly announced plans would allow third parties to manufacture such drugs if manufacturers make them unaffordable.

Pharma Times
DECEMBER 6, 2023
The neurological condition currently affects one in 300 people in the UK - News - PharmaTimes

Bio Pharma Dive
DECEMBER 4, 2023
The acquisition of Carmot, which had been planning an IPO, will hand Roche three weight loss medicines in early clinical testing.
pharmaphorum
DECEMBER 4, 2023
Uveal melanoma: A new generation of therapies offers hope for patients Mike.
Let's personalize your content