A unified, connected foundation is transforming the future of digital trials
Bio Pharma Dive
NOVEMBER 21, 2022
A holistic approach allows stakeholders to adapt to the ever-increasing number of clinical trials and data points.

Bio Pharma Dive
NOVEMBER 21, 2022
A holistic approach allows stakeholders to adapt to the ever-increasing number of clinical trials and data points.
Pharmaceutical Technology
NOVEMBER 25, 2022
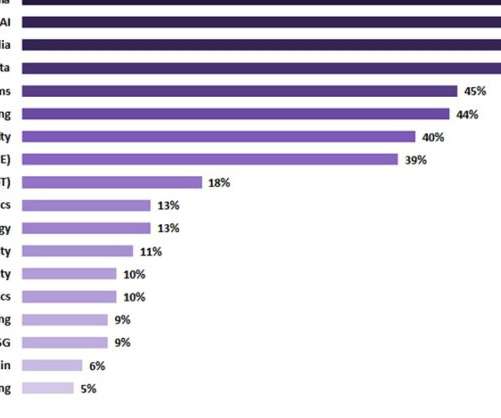
Artificial intelligence (AI) was seen as one of the top current investment priorities and was thought to continue to attract investment in the healthcare sector in the upcoming two years, according to GlobalData's latest report ‘Digital Transformation and Emerging Technology in the Healthcare Industry - 2022 Edition’. In this survey-based report tracker, digital media was prioritised as a top current investment target, with 53% of surveyed respondents confirming that their companies are currentl
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 22, 2022
In recent years, a growing number of scientific studies have backed an alarming hypothesis: Alzheimer’s disease isn’t just a disease, it’s an infection. While the exact mechanisms of this infection are something researchers are still trying to isolate, numerous studies suggest the deadly spread of Alzheimer’s goes way beyond what we used to think.

Medical Xpress
NOVEMBER 24, 2022
A team of researchers affiliated with multiple institutions in China, working with a colleague in the U.S., has isolated a type of bacteria in the guts of mice that break down nicotine. In their paper published in the journal Nature, the group describes how they isolated the bacteria and why their finding could reduce incidences of fatty liver disease in humans.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Bio Pharma Dive
NOVEMBER 22, 2022
The treatment, which is for the less common “B” form of the bleeding disorder, will be sold in the U.S. by maker CSL for $3.5 million.

Pharmaceutical Technology
NOVEMBER 25, 2022
In recent months, Wegovy (semaglutide), indicated for obesity, has been subjected to widespread supply shortages due to high demand, in addition to manufacturer production problems, with Novo Nordisk intending to relaunch Wegovy by the end of 2022. In the meantime, however, Novo Nordisk’s Ozempic, another version of semaglutide indicated particularly for patients with type 2 diabetes, has picked up the slack by being used off-label for obesity patients.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

STAT News
NOVEMBER 22, 2022
A viral hurricane is making landfall on health care systems battered by three pandemic years. With the official start of winter still weeks away, pediatric hospitals are facing crushing caseloads of children sick with RSV and other viral illnesses. Schools that promised a “return to normal” now report widespread absences and even closures from RSV and flu in many parts of the country , contributing to parents missing work in record numbers.

Bio Pharma Dive
NOVEMBER 21, 2022
Eight months after Daiichi Sankyo shuttered the San Francisco Bay Area subsidiary, Plexxikon’s former CEO is resuscitating its drug research under a new name.

Pharmaceutical Technology
NOVEMBER 20, 2022
The UK's National Institute for Health and Care Excellence (NICE) has published a draft guidance that does not recommend the use of five major Covid-19 therapies. This news came as many organisations and patient advocacy groups have been campaigning for the adoption of some of the rejected drugs for several months, amidst concerns about access to medicines in the UK.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 23, 2022
The mental fog that can come with pregnancy – commonly referred to as ‘baby brain’ – isn’t merely the result of discomfort, stress, and sleepless nights: A mother’s brain really does seem to change to accommodate the new arrival. A new study by researchers from the Netherlands has uncovered strong evidence of a relationship between […].

BioPharma Reporter
NOVEMBER 21, 2022
A Phase 3 trial for Northwest Biotherapeuticsâs DCVax-L vaccine extended survival in patients with gliobastoma for many months, or in some cases, years: according to new data.

Bio Pharma Dive
NOVEMBER 22, 2022
The British drugmaker has begun the process of withdrawing its multiple myeloma treatment Blenrep following a request from the FDA.
Pharmaceutical Technology
NOVEMBER 22, 2022
Umoja Biopharma has signed a research agreement with IASO Biotherapeutics (IASO Bio) to develop off-the-shelf therapies for haematological malignancies. Under the alliance, the companies will assess the induced cytotoxic innate lymphocytes (iCIL) platform of Umoja with chimeric antigen receptors (CARs) of IASO to develop the next generation of widely accessible, easily available cell therapies.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 22, 2022
Drug manufacturers have hailed the Drugs (Eighth Amendment) Rules, 2022 mandating barcode or quick response (QR) code on the label of top 300 brands of formulations from August 1, 2023, saying that QR codes will help identify misbranded or counterfeit medicines as well as recall the products if its quality gets compromised during manufacturing. Welcoming […].

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

NPR Health - Shots
NOVEMBER 20, 2022
The treatments were highly popular earlier in the pandemic. One by one, they got knocked out by more convenient, less expensive treatment options, and new COVID variants.

Bio Pharma Dive
NOVEMBER 21, 2022
Our latest research uncovers new insights on how brands can truly close the wellness gap and grow within their industry sectors.

Pharmaceutical Technology
NOVEMBER 23, 2022
The US Food and Drug Administration (FDA) has granted approval for CSL Behring’s adeno-associated virus vector-based gene therapy, Hemgenix (etranacogene dezaparvovec), to treat haemophilia B (congenital Factor IX deficiency) in adult patients. The treatment is indicated for usage in such individuals who use Factor IX prophylaxis treatment, or have an existing or historical life-threatening haemorrhage, or recurrent, serious episodes of sudden bleeding.

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 23, 2022
The Indian Pharmacopoeia Commission (IPC) has rejected Central Drugs Standard Control Organisation (CDSCO)’s request for extension of timelines to implement Indian Pharmacopoeia (IP) 2022. Drugs Controller General of India (DCGI) Dr VG Somani on October 28, 2022 wrote to Dr Rajeev Singh Raghuvanshi, secretary-cum-scientific director, IPC urging him to extend the deadline for implementation of […].

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

NPR Health - Shots
NOVEMBER 21, 2022
Taxpayers footed the bill for care that should have cost far less, according to records released under the Freedom of Information Act. The U.S. government may charge insurers $650 million as a result.

Bio Pharma Dive
NOVEMBER 21, 2022
Richard Francis will take over for the retiring Kåre Schultz on Jan. 1. He’ll face major challenges at Teva, including opioid settlements, biosimilar launches and reducing the company’s debt.

Pharmaceutical Technology
NOVEMBER 25, 2022
Japan’s Ministry of Health, Labour and Welfare (MHLW) has granted approval for Daiichi Sankyo ’s Enhertu (trastuzumab deruxtecan) to treat HER2-positive unresectable or recurrent breast cancer in adults. The treatment is indicated for usage in such patients following previous chemotherapy, comprising trastuzumab and a taxane. The latest development was based on the findings from the international, head-to-head, open-label, randomised Phase III DESTINY-Breast03 trial which analysed the efficacy a

AuroBlog - Aurous Healthcare Clinical Trials blog
NOVEMBER 24, 2022
The Central Drugs Standard Control Organisation (CDSCO) has approved four more Medical Device Testing Laboratories (MDTL) to carry out tests or evaluation of a medical device on behalf of the manufacturers under the provisions of the Medical Devices Rules, 2017. With this, the total number of MDTLs approved by the regulator is 28 across the […].

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
BioPharma Reporter
NOVEMBER 22, 2022
GeNeuro has recruited the first patients in a Phase 2 trial evaluating temelimab against long-COVID: assessing the efficacy of the treatment for improving cognitive impairment and fatigue.

Bio Pharma Dive
NOVEMBER 21, 2022
The deal is Merck’s second notable acquisition of a cancer drugmaker in the past year and a half, part of a strategy to diversify its pipeline beyond top-selling immunotherapy Keytruda.

Pharmaceutical Technology
NOVEMBER 25, 2022
XtalPi has entered a strategic partnership with CK Life Sciences for artificial intelligence (AI)-driven tumour vaccine research and development (R&D). Under the collaboration, the companies will utilise their capabilities to co-develop a new AI tumour vaccine R&D platform. Such a platform will aid in boosting tumour vaccine discovery and design expertise as well as expedite the development of other kinds of vaccines.

Medical Xpress
NOVEMBER 24, 2022
Some wounds just won't heal. Infections, diseases like diabetes, and suppressed immune systems often stack up to slow healing. Chronic wounds can last months and lead to anxiety and depression. In the worst cases, they are life threatening. Cost of treatment has soared to $25 billion each year.

NPR Health - Shots
NOVEMBER 22, 2022
As the holiday approaches, infectious disease specialists are bracing for the possibility that big family get-togethers and travel will propel the spread of RSV, flu and COVID-19.

Bio Pharma Dive
NOVEMBER 22, 2022
Over the past two decades, the Cambridge area has become a nerve center for biotech in the U.S. But to stay relevant and accessible, the hub is expanding to the suburbs.

Pharmaceutical Technology
NOVEMBER 22, 2022
The Ministry of Health, Labour and Welfare (MHLW) in Japan has granted emergency regulatory approval for Shionogi ’s new anti-SARS-CoV-2 drug, Xocova (ensitrelvir fumaric acid, S-217622), for Covid-19. This approval under the emergency regulatory approval system is granted under the Article 14-2-2 of the Pharmaceuticals and Medical Devices Act, the company noted.

Medical Xpress
NOVEMBER 24, 2022
A team of researchers at a company called Contraline has developed a new kind of male contraceptive. Instead of using hormones to disrupt sperm production, the new technique involves placing a hydrogel called ADAM into the vas deferens to prevent sperm from making its way to the urethra. The new technique is not yet available for men seeking an alternative way to prevent pregnancy, however—it is currently undergoing a clinical trial in Australia.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Let's personalize your content