How are digital biomarkers reshaping Parkinson’s disease research?
Bio Pharma Dive
JANUARY 30, 2023
Your Parkinson’s disease trials need better data. Hear how digital biomarkers change the game.

Bio Pharma Dive
JANUARY 30, 2023
Your Parkinson’s disease trials need better data. Hear how digital biomarkers change the game.
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections. This marks the second time one of Janssen’s HIV vaccines failed after another showed disappointing results in the Phase IIb Imbokodo trial in August 2021.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 31, 2023
The average temperature of the human body has been steadily declining since the middle of the 19th century, and scientists aren’t sure why. A new study suggests one key factor that might play a role in this: gut microbes.

Medical Xpress
FEBRUARY 3, 2023
Cinnamon, the well-known aromatic spice that many of us use to bake cakes and cook savory dishes, is derived from the inner bark of Cinnamomum trees. These are evergreen trees found in the Himalayas and other mountain areas, as well as in rainforests and other forests in southern China, India and Southeast Asia.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
FEBRUARY 2, 2023
The company’s lead drug could be an oral alternative to marketed diabetes and obesity drugs from Novo Nordisk and Eli Lilly.
Pharmaceutical Technology
JANUARY 31, 2023
Gene therapy company uniQure has entered into a global licensing agreement with Apic Bio for APB-102 to treat patients with amyotrophic lateral sclerosis (ALS) caused by mutations in superoxide dismutase 1 (SOD1). Under the terms of the deal, uniQure will obtain the worldwide rights to develop and market the clinical stage gene therapy, APB-102. uniQure stated that the license of APB-102 further strengthens its gene therapies pipeline developed for the treatment of neurological disorders as well
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

STAT News
FEBRUARY 1, 2023
A recent study and accompanying news story in the preeminent journal Nature provocatively concludes that disruptive innovation in science has dramatically and mysteriously declined 90% since 1945. The study has prompted a wave of news coverage and tweets decrying the apparent languishing of modern science. We feel that the authors make interesting observations on publishing trends, but their conclusions seem to be quite disconnected from the valuable and transformative innovations that benefit h

Bio Pharma Dive
FEBRUARY 1, 2023
The agency is currently assessing applications filed by the two companies for what they hope will become the first vaccines against the virus in older adults.

Pharmaceutical Technology
FEBRUARY 2, 2023
The US Food and Drug Administration (FDA) has approved GlaxoSmithKline ’s (GSK) Jesduvroq (daprodustat) to treat anaemia caused by chronic kidney disease (CKD) in adults who have been on dialysis for at least four months. Jesduvroq is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI). It is claimed to be the only HIF-PHI approved in the country that offers a new oral treatment option for adult patients on dialysis with anaemia of CKD.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 1, 2023
A study of around 500,000 medical records has suggested that severe viral infections like encephalitis and pneumonia increase the risk of neurodegenerative diseases like Parkinson’s and Alzheimer’s. Researchers found 22 connections between viral infections and neurodegenerative conditions in the study of around 450,000 people.

Fierce Pharma
FEBRUARY 3, 2023
J&J's pharma group quietly works through global overhaul, with layoffs expected to reach multiple countries mbayer Fri, 02/03/2023 - 09:29

Bio Pharma Dive
JANUARY 30, 2023
The report, a yearly poll of 100 financing executives by accounting and consulting firm BDO, revealed the many levers young drugmakers are pulling to conserve cash.

Pharmaceutical Technology
FEBRUARY 1, 2023
Pfizer has reported a 30% operational growth in revenues to $100.3bn in full-year 2022 compared with $81.2bn reported last year. The revenues grew 2% operationally excluding Paxlovid and Comirnaty’s contributions. In the fourth quarter (Q4) of 2022, they increased 13% operationally to $24.3bn compared to $23.8bn in the same quarter last year. On an operational basis, the revenues rose by 5%, omitting contributions from Paxlovid and Comirnaty.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 29, 2023
The hypertension drug rilmenidine has been shown to slow down aging in worms, an effect that in humans could hypothetically help us live longer and keep us healthier in our latter years. Rilmenidine was picked for this latest study because past research has shown it mimics the effects of caloric restriction on a cellular level.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

STAT News
JANUARY 30, 2023
The federal government will audit Medicare Advantage insurers aggressively under a rule finalized Monday, which is expected to result in billions of dollars in overpayments going back toward Medicare’s trust fund and patients over the next decade. However, federal officials watered down one of the auditing policies by giving insurers seven years of immunity from having the samples of their diagnosis coding errors extrapolated to their broader Medicare Advantage membership.

Bio Pharma Dive
JANUARY 30, 2023
The agency’s clearance of Jaypirca gives Lilly another win from its Loxo buyout, while Menarini Group’s bet on Radius Health’s oral SERD has now paid off with Orserdu’s OK.
Pharmaceutical Technology
FEBRUARY 2, 2023
If things go as per plan, in a few months, the US Food and Drug Administration (FDA) will deliberate on the first-of-its-kind CRISPR-based gene therapy for sickle cell disease (SCD) and transfusion-dependent beta thalassemia. Having made significant advances in a relatively short period of time, CRISPR research is now edging closer to reaching the clinic , and this month’s cover story takes a look at the major players in this field and the big events that could break through this year.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 30, 2023
For more than a century, the womb has been largely considered a sterile environment. Yet even today, with advanced medical technology at hand, researchers cannot come to a consensus over whether the placenta and the amniotic fluid that bathes a fetus are truly germ-free or not.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

NPR Health - Shots
FEBRUARY 3, 2023
The main reason the surge is ebbing now, pandemic experts suspect, is the significant immunity many people in the U.S. have acquired from prior infections and COVID vaccinations many received.

Bio Pharma Dive
FEBRUARY 3, 2023
The biotech recently stopped a trial of its experimental conditioning regimen over safety concerns. Now it’s halting further development work as it undertakes a strategic review.

Pharmaceutical Technology
FEBRUARY 3, 2023
Merck (MSD outside North America) has reported a 2% increase in worldwide sales to $13.83bn in the fourth quarter (Q4) of 2022 from $13.52bn in the previous year’s quarter. The company’s pharmaceutical sales recorded a 1% rise to $12.18bn compared to $12.03bn in the prior-year quarter. For the quarter, GAAP earnings per share (EPS) from continuing operations was $1.18, indicating a 22% decline from $1.5 in the same quarter last year.

AuroBlog - Aurous Healthcare Clinical Trials blog
JANUARY 31, 2023
The Ghaziabad-based Indian Pharmacopoeia Commission (IPC) which is the national co-coordinating centre (NCC) for the Materiovigilance Programme of India (MvPI) has identified 119 new Medical Device Adverse Event Monitoring (MDAEM) centres in various states to report medical device adverse events.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharma Times
FEBRUARY 1, 2023
UK-wide therapy for patients who have seizures linked to tuberous sclerosis complex

Bio Pharma Dive
FEBRUARY 3, 2023
The company, which worked with GSK to develop a coronavirus shot, is ceasing operations after its corporate parent cut off further investment.

Pharmaceutical Technology
FEBRUARY 3, 2023
The field of genomic medicine has reached a true turning point. With scientists fervently developing mRNA vaccines, nucleic acid therapeutics, and viral vector-based gene therapies, clinicians are set to have a growing number of tools available to treat a wide range of conditions, from infectious diseases to genetic disorders and more. After years of safety-related setbacks and development challenges, recent progress in gene therapy is especially exciting.

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 1, 2023
Indian pharma and clinical research organisations are working to explore the potential usage of ChatGPT in pharmacovigilance, the science of monitoring the safety of medications. ChatGPT is a chatbot launched by the US-based OpenAI in November 2022. This tool can be used across the pharmaceutical industry and its related service providers.

NPR Health - Shots
JANUARY 31, 2023
Children ages 19 and under died from COVID-19 at a rate at 1 per 100,000, making it rare, but still a leading cause of death among that age group.

Bio Pharma Dive
JANUARY 31, 2023
The pipeline of CAR-T therapies and ex vivo gene therapies has swelled in recent years, but manufacturing hasn’t been able to keep up with demand.
Pharmaceutical Technology
JANUARY 30, 2023
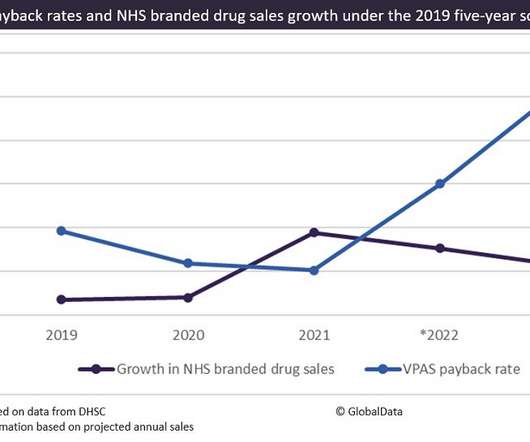
The UK government’s decision to set an unprecedented payback rate of 26.5% on National Health Service (NHS) sales of branded medicines in 2023, almost double that of the previous year, has sent shock waves through the pharma industry. This payback rate applies to corporate members of the Voluntary Scheme for Branded Medicines Pricing and Access (VPAS), a five-year scheme initiated in 2019, which returns a proportion of funds to the NHS based on the sales of branded prescription medicines (innova

AuroBlog - Aurous Healthcare Clinical Trials blog
FEBRUARY 2, 2023
The Subject Expert Committee (SEC) that advises the drug regulator of the country has recommended waiver of local phase III and IV clinical trials in India for Sanofi Healthcare India’s orphan drug to treat the Pompe disease. The Committee recommended grant of permission to import and market the drug without these stages of studies.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Let's personalize your content