FDA approves first gene therapy for hemophilia B
Bio Pharma Dive
NOVEMBER 22, 2022
The treatment, which is for the less common “B” form of the bleeding disorder, will be sold in the U.S. by maker CSL for $3.5 million.
Bio Pharma Dive
NOVEMBER 22, 2022
The treatment, which is for the less common “B” form of the bleeding disorder, will be sold in the U.S. by maker CSL for $3.5 million.

Pharma Mirror
DECEMBER 16, 2022
The COVID-19 pandemic challenged researchers to discover life-saving treatments at an unprecedented pace. While these researchers rose to the occasion by discovering drugs faster than ever before, variants continue to challenge the world in addressing COVID-19. New innovations in computer-aided drug discovery tools could dramatically accelerate the study of new variants.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

World of DTC Marketing
AUGUST 15, 2022
The great resignation has not affected pharma because the jobs within the industry pay very well and most positions and employees like the benefit of having a steady job with good money. However, some pharma companies are having problems filling open positions, and when they do, they often find it wasn’t a good fit. Here are some things to consider to ensure you’re hiring good people.
Pharmaceutical Technology
NOVEMBER 25, 2022
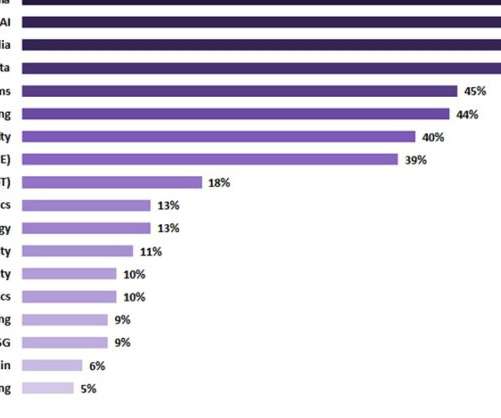
Artificial intelligence (AI) was seen as one of the top current investment priorities and was thought to continue to attract investment in the healthcare sector in the upcoming two years, according to GlobalData's latest report ‘Digital Transformation and Emerging Technology in the Healthcare Industry - 2022 Edition’. In this survey-based report tracker, digital media was prioritised as a top current investment target, with 53% of surveyed respondents confirming that their companies are currentl

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Bio Pharma Dive
MARCH 24, 2022
At least nine biotechs working in cell or gene therapy have announced layoffs, cost cuts or restructured their research since December — restructurings that have coincided with a stock market downturn.

World of DTC Marketing
JANUARY 28, 2022
A cancer medication called Xtandi costs $189,800 per year and was developed with taxpayer dollars. The U.S. government has a responsibility for ending the exclusive patents that give them their profits. The Department of Health and Human Services is currently considering whether to allow the generic manufacturing of Xtandi, which could drop the price of a pill from $400 to $3 overnight.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Bio Pharma Dive
NOVEMBER 29, 2022
At a medical conference, the companies detailed clinical trial results that could help support approval of their drug, lecanemab. However, some doctors aren’t yet convinced the medicine’s risks are worth its potential benefits.

World of DTC Marketing
JULY 15, 2022
To address the “retention” problem, pharma companies are paying employees exorbitant salaries. The problem with that system is that people do whatever they need to to “hold onto” their high-paying jobs and become employees who are content not to excel. This is NOT what pharma needs. Last week I briefly chatted with someone in the industry evaluating another job offer.
Bio Pharma Dive
SEPTEMBER 28, 2022
The companies said the drug, called lecanemab, met all of its goals in the Phase 3 study. The data are a significant finding and provide stronger support for a much-debated hypothesis for treating Alzheimer’s.

Bio Pharma Dive
SEPTEMBER 30, 2022
The drug, which will be sold as Relyvrio, showed modest benefits in function and survival in testing. It also became the latest test of the FDA's flexibility toward new therapies for neurological disorders.
Bio Pharma Dive
NOVEMBER 3, 2022
In a panel discussion hosted by BioPharma Dive, venture capitalists and CEOs discussed how startups can navigate a challenging market as well as possible ripple effects from the new U.S. drug pricing law.

Pharmaceutical Technology
NOVEMBER 14, 2022
In a field dominated by antibodies and small molecules, two cell-therapy based approaches have come under the spotlight for showing early signs of efficacy in treating lupus. In September, a group from Friedrich Alexander University Erlangen-Nuremberg reported that five patients with lupus achieved remission after an infusion of autologous chimeric antigen receptor (CAR)-T cells led to a deep depletion of B cells.

Bio Pharma Dive
AUGUST 17, 2022
The regulator cleared the biotech’s medicine Zynteglo for transfusion-dependent beta thalassemia, giving patients a powerful new treatment option. But it will come at a very high cost of $2.8 million in the U.S.
Bio Pharma Dive
AUGUST 3, 2022
The success of Alnylam's treatment in a trial known as APOLLO-B could pave the way for the biotech to reach consistent profitability, should the FDA sign off on a planned approval application.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Bio Pharma Dive
NOVEMBER 14, 2022
The deal, which could carry a total value of roughly $145 million, is in large part focused on a nasal formulation of the opioid overdose drug nalmefene.

Bio Pharma Dive
JULY 26, 2022
New companies are being built more carefully and platform technologies are less in vouge as the downturn's effects ripple through the private sector.

Bio Pharma Dive
OCTOBER 18, 2022
The University of Pennsylvania immunologist and inventor of Kymriah spoke with BioPharma Dive about working with pharma, starting companies and the future of the cell therapy field.
Bio Pharma Dive
OCTOBER 18, 2022
At least five startups have emerged with new ways to genetically modify immune cells within the body, an approach that, if successful, could widen the field of CAR-T treatment.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Bio Pharma Dive
JUNE 15, 2022
A closely watched study missed its goal, failing to prove the antiviral pill’s benefit in a broader population than the high-risk individuals for which it’s currently cleared.

Bio Pharma Dive
DECEMBER 1, 2022
The regulatory OK, a milestone for microbiome-based drug research, is for a medicine from Ferring Pharma that treats a recurrent type of gut infection.

Bio Pharma Dive
FEBRUARY 6, 2022
For the first time in years, biotechs no longer have an easy path onto Wall Street, a market reversal that could change what the next generation of young drugmakers looks like.

Bio Pharma Dive
FEBRUARY 28, 2022
The CAR-T treatment is the second to be approved in the U.S. for the blood cancer, following Bristol Myers Squibb and 2Seventy bio's Abecma.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Bio Pharma Dive
JANUARY 2, 2022
Biotech stocks ended 2021 in a slump. But positive results from eagerly anticipated studies in breast cancer, schizophrenia and Alzheimer's disease could help turn the sector's fortunes around.

Bio Pharma Dive
JANUARY 12, 2022
Analysts expect that deal engines are ready to start firing again following the recent quiet period. Still, uncertainty remains about how biopharma acquisitions could play out in the coming months.

Bio Pharma Dive
JANUARY 10, 2022
Pfizer's work with mRNA vaccines led it explore other applications of the technology, resulting in a multi-year partnership with the high-profile biotech Beam Therapeutics on gene editing treatments for rare diseases.
Bio Pharma Dive
OCTOBER 6, 2022
Nested Therapeutics touts a deep bench of scientific leaders and advisers, including Kevan Shokat, whose work drugging KRAS — a cancer-related gene once thought to be undruggable — helped lead to the development of Amgen’s Lumakras.

Advertiser: FourKites
A research study conducted by The Journal of Commerce and FourKites surveyed hundreds of international shippers, exploring how their usage of global supply chain visibility technology has evolved since the onset of global disruptions caused by COVID-19. For international shippers, ocean freight visibility has evolved from optional to essential and satisfaction with visibility varies greatly depending on how it is obtained and delivered.

Bio Pharma Dive
SEPTEMBER 17, 2022
The therapy, called Skysona and cleared to treat cerebral adrenoleukodystrophy, is the product of more than a decade of work by Bluebird. It will cost $3 million.

Bio Pharma Dive
JANUARY 19, 2022
Hal Barron, a key figure in GSK's efforts to revitalize its drug research, will step down as its top scientist at a time when the company is feeling heat from investors to deliver faster growth.

Bio Pharma Dive
APRIL 14, 2022
Fresh trial results intensify competition with Roche to deliver the first lymphoma drug that marries antibody technology with an immunotherapy pathway.

Bio Pharma Dive
SEPTEMBER 30, 2022
The application comes a year earlier than previously had been expected, as company says drug reviewers are open to accelerated review based on data from early-stage trials.

Speaker: Steve Goldstein, Sales Leader
Are you currently in sales, or involved in a business that depends on strong sales results? What about the extremely competitive world of medical device sales? What are some of the top challenges your customers face and how do you approach understanding what’s most important to them? Join Steve Goldstein, Sales Success Coach, Motivational Speaker and Medical Device Sales Leader from Gold Selling LLC., to discover critical strategies and approaches you can take to engage your customers, achieve g
Let's personalize your content