Ginkgo grows its gene therapy offerings with StrideBio deal
Bio Pharma Dive
APRIL 5, 2023
The deal hands Ginkgo technology for discovering and engineering capsids — the outer shell that protects the helpful genetic material in gene therapies.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
APRIL 5, 2023
The deal hands Ginkgo technology for discovering and engineering capsids — the outer shell that protects the helpful genetic material in gene therapies.
Pharmaceutical Technology
JANUARY 5, 2023
Capsida Biotherapeutics and Eli Lilly and Company ’s wholly owned subsidiary Prevail Therapeutics have announced a partnership for the development of non-invasive gene therapies for central nervous system (CNS) diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 25, 2023
ElevateBio has raised $401m in a Series D financing round for advancing its technology platforms to expedite the design, production and development of cell and gene therapies. ElevateBio intends to use the funds to advance its genetic medicine current good manufacturing practice (cGMP) and process development business, BaseCamp.

Pharmaceutical Technology
APRIL 6, 2023
Ginkgo Bioworks has strengthened its end-to-end R&D capabilities in gene therapy with the purchase of adeno-associated virus (AAV) capsid discovery and engineering assets from StrideBio, for an undisclosed sum. The new capabilities and IP will be incorporated under Ginkgo’s end-to-end AAV gene therapy development platform.

Pharmaceutical Technology
FEBRUARY 24, 2023
AbbVie is expanding its strategic collaboration with Capsida Biotherapeutics for the development of targeted genetic medicines to treat eye diseases with high unmet needs. In pursuing the promise of genetic medicine-based therapeutics, AbbVie continues to expand our capabilities, and we are pleased to have Capsida as a partner.”

pharmaphorum
JUNE 15, 2021
When Biogen acquired Nightstar Therapeutics in 2019, it added a pair inherited retinal disorder (IRD) gene therapies that it hoped would accelerate a push into the fast-evolving category. . The post Biogen’s ambitions in gene therapy hit by another failed pivotal trial appeared first on.
Pharmaceutical Technology
APRIL 20, 2023
Circio aims to develop new circRNA medicines initially for cancer, then plans to expand rapidly into vaccines and gene therapy. The circVec platform is a modular genetic cassette that provides instructions for the generation of multifunctional circRNAs. Topic sponsors are not involved in the creation of editorial content.

Pharmaceutical Technology
MAY 17, 2023
The deal will see the integration of Scribe’s new CRISPR by Design approach and Prevail’s expertise in developing genetic medicines for neurological disorders for specific genetic targets. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
pharmaphorum
JANUARY 3, 2023
As December 2022 closed out, Pfizer announced positive top-line results from its phase 3 BENEGENE-2 study evaluating fidanacogene elaparvovec (SPK-9001), its investigational gene therapy for treatment of adult males with moderately severe to severe haemophilia B.
STAT News
JULY 11, 2022
Twenty-three years ago, the field of gene therapy was bursting with the promise of breakthrough treatments. Then it was almost instantly derailed by the death of an 18-year-old clinical trial volunteer named Jesse Gelsinger after he received a genetically engineered virus that had been developed to treat his rare liver condition.

Pharmaceutical Technology
FEBRUARY 13, 2023
Several biotech companies and researchers are now exploring medical devices and gene therapies to address not just common forms of epilepsy, but also rare conditions such as Dravet Syndrome. A genetic treatment for epilepsy Most treatments for epilepsy aim to treat seizures, which is the main symptom of this condition, says Ferraro.

STAT News
AUGUST 18, 2022
Food and Drug Administration approved a new treatment that delivers a potentially permanent, genetic fix for patients with the inherited blood disorder beta thalassemia — and quite possibly a financial lifeline for its manufacturer, Bluebird Bio , STAT tells us.
Pharmaceutical Technology
JUNE 4, 2023
Innovation S-curve for the pharmaceutical industry Transcription factors for AAV is a key innovation area in pharmaceutical Adeno-associated virus (AAV) vectors are widely used for gene therapy. There are two main genes in the AAV genome, rep and cap, which encode nine different proteins.

Pharmaceutical Technology
JUNE 29, 2022
For cell and gene therapy applications, you need a variety of speciality enzymes of the highest purity, specificity, and consistency. Novozymes has a long legacy of enzyme discovery and the ability to genetically engineer these speciality enzymes to be superior. And it’s within the family – Novozymes!”.
Advarra
JUNE 12, 2023
Gene therapy research is exciting and full of promise, but because of the risks involved, it’s also highly regulated, requiring an institutional biosafety committee (IBC) to provide additional oversight and risk assessment. What Does an IBC Review? How is IBC Membership Composed?

Pharmaceutical Technology
JANUARY 10, 2023
Asklepios BioPharmaceutical has entered a research partnership and option agreement with ReCode Therapeutics for exploring its single-vector gene-editing platform. Beyond the liver, ReCode’s SORT LNP genetic medicines technology enables the delivery to target cells and organs. By Cytiva Thematic.
Advarra
MARCH 31, 2023
The use of engineered genetic materials in clinical trials is rapidly expanding, with potential applications for genetic vaccines, gene-modified cellular therapies, and gene therapies. A key part of the IBC’s evaluation is assessing the risks posed by the engineered genetic materials.

Advarra
AUGUST 8, 2023
Gene therapy research is booming in the clinical setting. In this blog, we summarize the growth, risks, and regulatory requirements for gene therapy research. Defining the Boom in Gene Therapy Research The gene therapy field is experiencing explosive growth in today’s competitive research environment.

BioSpace
MAY 10, 2021
Biogen and Capsigen forged a strategic collaboration to engineer novel adeno-associated virus (AAV) capsids that have the potential to become transformative gene therapies that treat underlying genetic causes of various central nervous system and neuromuscular disorders.

Velocity Clinical Research
OCTOBER 3, 2024
As Nick Spittal states in this Advarra press release, membership in the Gene Therapy Ready (GTR) site network “allows Velocity to start studies over a month faster and provides a meaningful credential and important validation that increases sponsors’ confidence in our specialized capabilities to conduct complex clinical research safely.”

pharmaphorum
JANUARY 29, 2021
Since that discovery, a flurry of gene-editing focused biopharma companies have launched – including Intellia Therapeutics, CRISPR Therapeutics, Caribou Biosciences and Mammoth Biosciences – and the first drug therapies based on the technology are now in human testing for diseases like cancer. Macrae explains. “So

Advarra
JUNE 13, 2024
Recombinant DNA technologies and genetically modified biological agents are being adapted for a wide scope of therapeutic applications, and their use is becoming increasingly common in clinical trials. How can I protect myself from exposure? What should I do if I’m exposed?
Pharmaceutical Technology
APRIL 5, 2023
But while companies continue studying allogeneic CAR-T therapies, including for their coveted use in solid tumours, such advancements remain challenging. More broadly however, several advancements are on the horizon for cell and gene therapies in 2023. AZ: Cell and gene therapies often come with a high price.
Pharmaceutical Technology
SEPTEMBER 29, 2022
Scribe Therapeutics and Sanofi have signed a strategic partnership to expedite the development of breakthrough clustered regularly interspaced short palindromic repeats (CRISPR)-based cell therapies for cancer. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Advarra
JUNE 13, 2023
Research in gene therapies and genetically engineered drugs and vaccines are growing exponentially, and will only continue to become more popular. The accelerating gene therapy market is expected to grow globally by 16.6% between 2020-2027.

Scienmag
MARCH 19, 2021
Treatment involving a single injection has long-lasting effects BOSTON – Researchers have used a genetic engineering strategy to dramatically reduce levels of tau–a key protein that accumulates and becomes tangled in the brain during the development of Alzheimer’s disease–in an animal model of the condition.

Cloudbyz
JULY 31, 2024
This revolution is enabling the growth of innovative biomarker-based precision medicine and cell and gene therapy, transforming both clinical research and post-market care. This allows for the selection of patients who are most likely to respond to a particular therapy, optimizing treatment efficacy and minimizing adverse effects.

Pharmaceutical Technology
AUGUST 17, 2022
Biopharmaceutical contract development and manufacturing organisation (CDMO) AGC Biologics has entered a strategic collaboration with RoosterBio to expedite the manufacturing of cell and exosome therapies. These capabilities comprise cell and exosome genetic engineering for expressing therapeutic targets.

Pharmaceutical Technology
AUGUST 25, 2022
The development and usage of innovative technologies, as well as expertise from the selection of cells and genetic modification to intelligent automation of production and information technology systems, will be part of the centre. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
Advarra
NOVEMBER 29, 2022
The field of cell and gene therapies (CGT) is constantly evolving, and there has been significant progress in this area of research. However, despite the promise of these therapies, the regulations governing them lag the science, which in turn hinders the clinical translation of these novel medicines.

Scienmag
JUNE 9, 2021
Credit: RCSI Scientists have developed polypeptide-based materials that act as effective vectors for delivering gene therapies. The first-of-its-kind platform enables the vectors to be adapted to suit the specific gene therapy cargo.

Pharmaceutical Technology
FEBRUARY 23, 2023
The collaboration will combine the mRNA platform of Moderna with the gene editing technologies suite, including the base editing capabilities of Life Edit for the development of curative therapies to treat challenging genetic diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
XTalks
FEBRUARY 25, 2025
Mirum Pharmaceuticals, a biotech innovator known for developing therapies for rare metabolic disorders, now has FDA approval for its new treatment, Ctexli (chenodiol) tablets the first and only medication approved for cerebrotendinous xanthomatosis (CTX) in adults.

Scienmag
DECEMBER 3, 2020
A new study has found that a novel T cell genetically engineered by University of Arizona Health Sciences researchers is able to target and attack pathogenic T cells that cause Type 1 diabetes, which could lead to new immunotherapy treatments.
Scienmag
MAY 11, 2021
Proof-of-principle research shows that genes can be accurately edited in cells throughout the body Credit: Zhiqian Li Researchers at the University of California San Diego have laid the groundwork for a potential new type of gene therapy using novel CRISPR-based techniques.

STAT News
NOVEMBER 4, 2022
The clinical trial was to be the first time anyone got a gene editing therapy for muscular dystrophy. It was the first time that a gene editing therapy was custom-made to address a single individual’s unique genetic mutation. Epigenome editing aims to dial the expression of genes up or down.

Scienmag
MARCH 1, 2021
The in-person Cell Culture Engineering XVII Conference has been postponed until October 16 – October 22, 2021 in Tucson, Arizona. To highlight the key role that the CCE community plays to free people worldwide from the shackles of the COVID-19 pandemic, we are planning two webinars during the original dates of the conference.

BioTech 365
AUGUST 11, 2020
FDA grants Orphan Drug Designation to Neurogene’s adeno-associated virus vector with engineered transgene encoding the human CLN7 gene NEW YORK–(BUSINESS WIRE)–Neurogene Inc.,

Advarra
AUGUST 10, 2023
Rapid growth in gene therapy is expected to receive additional support as the Food and Drug Administration (FDA) Center for Biologics Evaluation and Research (CBER) prepares to launch Operation Warp Speed for Rare Diseases. Peter Marks, head of FDA’s CBER – the organization responsible for regulating gene therapies.

Scienmag
JUNE 2, 2021
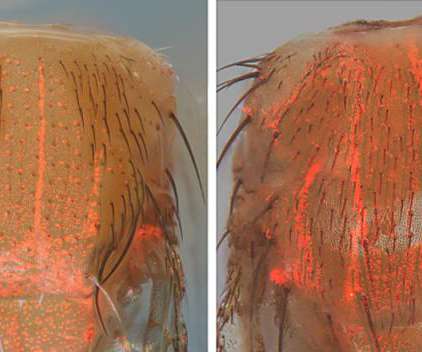
Researchers create novel CRISPR-based fly species as a new method of controlling gene drive spread Credit: Akbari lab, UC San Diego CRISPR-based technologies offer enormous potential to benefit human health and safety, from disease eradication to fortified food supplies.
XTalks
JANUARY 26, 2024
This technology, which allows for precise editing of DNA at specific locations, has been a major focus in the field due to its potential to directly target and modify cancer-causing genes. By editing these genes, researchers can effectively neutralize their cancer-promoting effects.

XTalks
SEPTEMBER 27, 2023
Poseida is a clinical-stage biopharmaceutical firm that utilizes its unique non-viral gene engineering methods to develop innovative cell and gene therapies. Scientists working at Poseida Therapeutics.

pharmaphorum
OCTOBER 4, 2022
AstraZeneca’s rare disease firm Alexion is set to expand its genomic medicine portfolio with the acquisition of gene editing specialist LogicBio Therapeutics, in a deal worth approximately $68 million.

XTalks
JANUARY 2, 2024
For instance, Vyjuvek , the first FDA-approved gene therapy for DEB, is priced at $24,250 per vial. a biotech company specializing in the development and commercialization of genetic medicines for rare diseases, announced FDA approval for Vyjuvek for the treatment of DEB. percent) compared to the control group (16.3
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content