Genetically modified herpes combats advanced cancers
Pharma Times
SEPTEMBER 21, 2022
A new genetically engineered virus has delivered a one-two punch in initial phase 1 trial
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
SEPTEMBER 21, 2022
A new genetically engineered virus has delivered a one-two punch in initial phase 1 trial
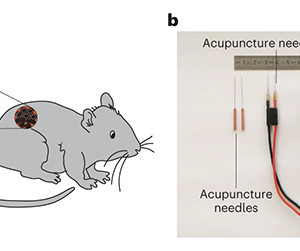
AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 7, 2023
New research from ETH Zürich in Switzerland could see future wearable devices (with perhaps a few implants and a touch of genetic engineering) boost our health directly. Fitness trackers help you stay healthy by keeping count of your steps and monitoring your heart rate, driving you on to hit those cardio goals.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
JUNE 10, 2023
CIGB-128 is under clinical development by Center for Genetic Engineering and Biotechnology and currently in Phase I for Brain Tumor. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is a combination of interferon alfa-2b with interferon gamma.

Pharmaceutical Technology
JUNE 10, 2023
CIGB-128 is under clinical development by Center for Genetic Engineering and Biotechnology and currently in Phase I for Brain Tumor. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is a combination of interferon alfa-2b with interferon gamma.

Pharmaceutical Technology
JUNE 24, 2022
The protein-based vaccine is engineered from the genetic sequence of the SARS-CoV-2 virus’ initial strain. The totality of preclinical, manufacturing and clinical trial findings that were submitted for analysis formed the basis for the EUA. The vaccine showed an encouraging safety and tolerability profile in both trials.

Pharmaceutical Technology
APRIL 6, 2023
Ginkgo Bioworks has strengthened its end-to-end R&D capabilities in gene therapy with the purchase of adeno-associated virus (AAV) capsid discovery and engineering assets from StrideBio, for an undisclosed sum. AAV is used in hundreds of active clinical trials, and is described as the preferred viral vector for gene therapy.
Pharmaceutical Technology
SEPTEMBER 5, 2022
In March last year, CanSinoBIO obtained approval for the clinical trial application to analyse Convidecia Air. Administered as a single dose, the genetically engineered vaccine has the replication-defective adenovirus type 5 vector that expresses the spike S protein of the SARS-CoV-2 virus.
Pharmaceutical Technology
MAY 10, 2023
SYNB1934 consumes Phe in the gastrointestinal (GI) tract by leveraging genetic engineering of the drug or drug-carrying capsule, probiotic escherichia coli (E coli) Nissle. This designation also comes at a pivotal time as we prepare to initiate our Phase 3 trial for PKU – Synpheny-3 – in the first half of 2023.”

Pharmaceutical Technology
APRIL 11, 2023
The finding was made as part of a study published in the journal Nature Biomedical Engineering. Kelley added: “Engineering-based tools allow you to do things that open up new areas of biology. CTRL Therapeutics will file an application with the US Food and Drug Administration (FDA) to advance the new platform into clinical trials.
Pharmaceutical Technology
JANUARY 5, 2023
Under the multi-year strategic partnership, Prevail will detect and advance capsids, which are clinically translatable, along with its cargo to develop the transformative genetic medicines by using Capsida’s novel adeno-associated virus (AAV) engineering platform.
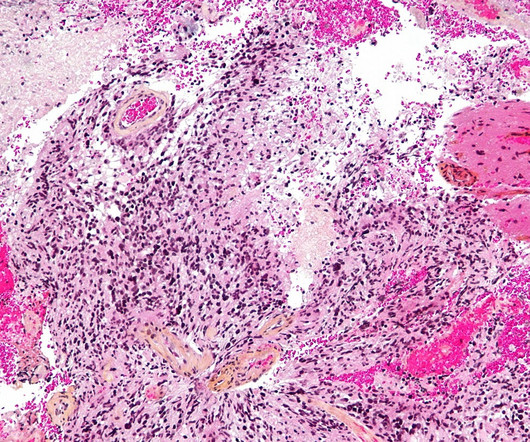
Pharmaceutical Technology
APRIL 26, 2023
This marks the first-ever designation for genetically modified gamma-delta T cell therapies. INB-400, an autologous, genetically engineered gamma-delta T cell therapy, is the company’s DeltEx chemotherapy-resistant autologous and allogeneic drug-resistant immunotherapy (DRI) technology.
BioSpace
APRIL 25, 2024
The genetic engineering startup, recently honored by BioSpace readers for its work environment, is downsizing as it seeks to launch its first clinical trials.

pharmaphorum
JUNE 15, 2021
Just over two years later, both of those candidates have failed late-stage clinical trials, leaving Biogen’s $800 million investment in Nightstar looking like a poor deal. The post Biogen’s ambitions in gene therapy hit by another failed pivotal trial appeared first on.

BioSpace
DECEMBER 6, 2021
Proof of concept for the Genetically Engineered Microbial Medicines (GEMM) platform came in a recent first-in-human, Phase I trial.

pharmaphorum
SEPTEMBER 11, 2022
It is the first time that a cell therapy for solid tumours has been tested in a phase 3 trial, and the first time that the approach has been directly compared with standard second-line immunotherapy in melanoma. ” The post ESMO: TIL therapy improves on Yervoy in melanoma trial appeared first on.

Pharmaceutical Technology
JUNE 29, 2022
In September 2021, GlobalData figures revealed there to be 1,320 industry-sponsored regenerative medicine and advanced therapy trials ongoing worldwide. Novozymes has a long legacy of enzyme discovery and the ability to genetically engineer these speciality enzymes to be superior. And it’s within the family – Novozymes!”.

Pharmaceutical Technology
NOVEMBER 29, 2022
The company now plans to move this approach into a Phase III trial. PharmaTher also has an agreement in place with Case Western Reserve University to develop ketamine as a treatment for Rett syndrome, a rare genetic neurological disorder. PharmaTher is not the only player in this space.
XTalks
FEBRUARY 25, 2025
CTX is a rare, progressive genetic disorder caused by mutations in the CYP27A1 gene, which disrupts the livers ability to produce chenodeoxycholic acid, a bile acid. The FDAs approval was supported by data from the Phase III RESTORE study a 24week, doubleblind, placebocontrolled, randomized crossover withdrawal trial.

pharmaphorum
MARCH 24, 2022
LifeMine has developed an industrialised, genomics-based discovery engine that it hopes will take a broad, systematic approach to identifying new compounds from fungi and screening them for activity against disease targets. Greg Verdine – LifeMine.

pharmaphorum
NOVEMBER 1, 2021
Delytact (teserpaturev) is a genetically engineered oncolytic herpes simplex virus type 1 (HSV-1) that was approved for marketing in Japan earlier this year, and received pricing approval in August at 1.43 million yen (around $12,500) per dose, according to a Pharma Japan report.
Pharmaceutical Technology
NOVEMBER 29, 2022
These viruses are engineered genetically for killing cancer. The researchers plan to seek the US Food and Drug Administration approval to commence clinical trials of the viral platform comprising chemokines and cetuximab, in humans. GBMs are called “cold” tumours as they lack helpful immune cells.

Pharmaceutical Technology
MARCH 2, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The therapeutic candidate comprises allogeneic NK cells genetically engineered to express chimeric antigen receptors (CAR-NK) targeting cells CD33.

Clinical Trial Podcast
FEBRUARY 3, 2024
To learn more about clinical trials in diabetes, I invited Dr. Stayce Beck, Global Vice President of Clinical Affairs at Dexcom Inc. in Chemical Engineering from the University of Texas at Austin. The post Diabetes Clinical Trials with Dr. Stayce Beck appeared first on Clinical Trial Podcast & Blog. on the podcast.

XTalks
AUGUST 29, 2022
CRISPR is notable for engineering living cells, allowing scientists to edit, turn off, delete, or replace genes in a cell’s genome. The goal of the team was to significantly increase yields of correctly engineered immune cells with CRISPR to effectively manufacture cell therapies. From the Bench to Patient Therapeutics.

XTalks
AUGUST 8, 2024
Tecelra is the first cell therapy engineered for a solid tumor cancer in the US, offering a new option for patients with specific genetic markers who have previously undergone chemotherapy. Clinical trial results for the therapy are expected to be shared in late 2024. The trial showed a 43 percent response rate with 4.5

Pharma Marketing Network
NOVEMBER 23, 2024
By leveraging research into specific genetic markers and tailored therapies, campaigns can address niche markets with unparalleled relevance. The Role of Real-World Evidence Real-world evidence (RWE) bridges the gap between clinical trials and everyday use. Authentic, research-backed messaging builds trust and credibility.
XTalks
NOVEMBER 23, 2020
Severe cases of the infection did not occur among trial participants, nor were any hospitalizations reported. The global trials are assessing the safety and efficacy of the vaccine in individuals aged 18 years or over from diverse racial and geographic groups who are healthy or have stable underlying medical conditions.

Scienmag
JUNE 14, 2021
Investments have catalyzed research, clinical trials and revolutionary treatments across many disciplines UC San Francisco is launching a new initiative to propel the development of living therapeutics – a category of treatments broadly defined as human and microbial living cells that are selected, modified, or engineered to treat or cure disease (..)

pharmaphorum
JANUARY 26, 2023
Yescarta – a CD19-directed genetically modified autologous T-cell immunotherapy investigated in the ZUMA-1 trial – is the first chimeric antigen receptor (CAR) T-cell therapy recommended for routine use on the NHS in England , meaning that for the first time eligible patients will be able to access CAR-T cell therapy in the long-term.
Pharmaceutical Technology
APRIL 5, 2023
And my estimate is that between 25,000 and 30,000 patients have been treated with those therapies globally, which does not include patients treated in clinical trials. We have the emergence of this new pillar of medicine, which is out of the lab and beyond clinical trials now. We must approach this on several fronts.

XTalks
OCTOBER 20, 2023
Clinical-stage genome editing company Intellia Therapeutics has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to start a pivotal phase III trial of NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis with cardiomyopathy.

Scienmag
MAY 28, 2021
These “liquid biopsy” analyses exploit the unique epigenetic landscape of bone tumors and do not depend on any genetic alterations, […]. The scientists analyzed short fragments of tumor DNA that are circulating in the blood.

XTalks
DECEMBER 20, 2023
This includes analyzing how drug combinations may impact individual patients or groups of patients before even entering a clinical trial. With a staggering 90 percent of drugs failing in clinical trials, AI has the potential to help improve these statistics. These companies are at various stages of research and clinical trials.

Advarra
AUGUST 8, 2023
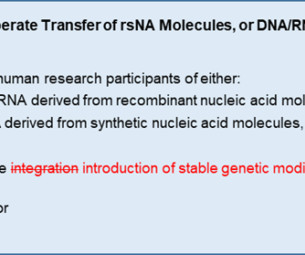
Gene therapy involves the transfer of engineered genetic materials to human research subjects. These studies were previously considered to be highly experimental and limited to early phase trials at a handful of highly specialized academic medical centers.
pharmaphorum
JANUARY 3, 2023
Haemophilia is a rare genetic bleeding disorder that causes the blood to take a long time to clot because of a deficiency in one or several blood clotting factors. SPK-9001 is a novel, investigation vector containing a bio-engineered adeno-associated virus (AAV) capsid (protein shell) and a high-activity human coagulation FIX gene.

XTalks
JUNE 2, 2022
XTALKS WEBINAR: Next-Gen Cell Therapies: Listening and Engaging with Patient Perspectives as an Essential Element in Clinical Trials. CAR-T is a type of immunotherapy in which the patients’ own T-cells are engineered to target and remove cancer cells. Kymriah Clinical Trial Results. x 10 8 CAR-positive viable T.

XTalks
JANUARY 2, 2024
XTALKS WEBINAR: Strategic Design Decisions in Rare Disease Trials Live and On-Demand: Thursday, February 8, 2024, at 11 am EST (4 pm GMT/UK) Register for this free webinar to discover invaluable strategies to effectively navigate rare disease trials, enhancing endpoint adaptation, data quality and clinical interpretation.
XTalks
JANUARY 26, 2024
By targeting and identifying specific genetic mutations, this technology could lead to the development of more precise diagnostic tools, enabling early intervention and treatment. By tailoring treatments based on an individual’s genetic makeup, it may allow for more effective and targeted therapies.
XTalks
JANUARY 21, 2025
From non-invasive cancer diagnostics to life-saving cardiovascular implants, the latest medical devices cleared or approved by the FDA in 2024 reflect the remarkable strides in science and engineering. There, plasma is separated, and tumor DNA is identified by detecting genetic mutations, methylation patterns and fragmentation signals.
WCG Clinical
NOVEMBER 17, 2023
Since then, however, certain genetic engineering technologies (e.g., For example, two trials testing genetically identical genome-edited cell therapies – one engineered with a viral vector, and one without – can differ in their IBC review requirements because of how the cells were modified rather than what they have become.

pharmaphorum
OCTOBER 19, 2022
PKU is a rare genetic disease that manifests at birth and is marked by an inability to break down phenylalanine (phe), an amino acid that is commonly found in many foods. Left untreated, high levels of the amino acid become toxic to the brain and may lead to serious neurological and neuropsychological issues.
XTalks
JULY 31, 2020
The study developed and researched microbe-controlled and genetically-engineered mice to understand the healing response in the intestine when receiving specific signals from bacteria. If we can influence these interactions, we may be able to control many diseases that are impacted by our microbiome or diet,” she says.

Cloudbyz
JULY 31, 2024
These technologies provide insights into genetic, proteomic, and metabolomic profiles, enabling the discovery of biomarkers associated with diseases like cancer, cardiovascular diseases, and neurological disorders. For instance, in oncology, patients with specific genetic mutations (e.g.,

The Pharma Data
NOVEMBER 30, 2020
CoV2373 is a stable, prefusion protein antigen derived from the genetic sequence of the SARS-CoV-2 coronavirus spike (S) protein and adjuvanted with Novavax’ proprietary Matrix?M. pivotal Phase 3 trial update. Novavax completed enrollment of 15,000 participants in a pivotal Phase 3 clinical trial being conducted in the U.K.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content