LegitScript Finds 1200 Percent Increase in Problematic Ads for Compounded GLP-1 Meds
XTalks
JULY 19, 2024
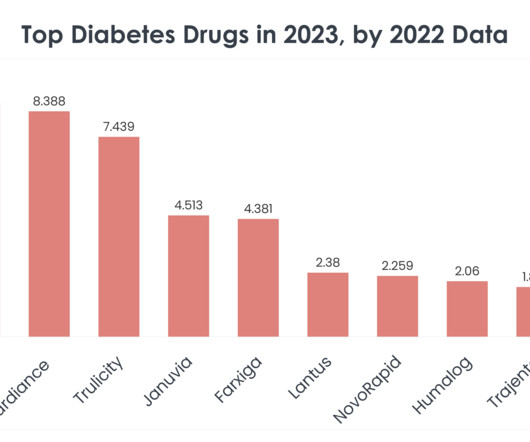
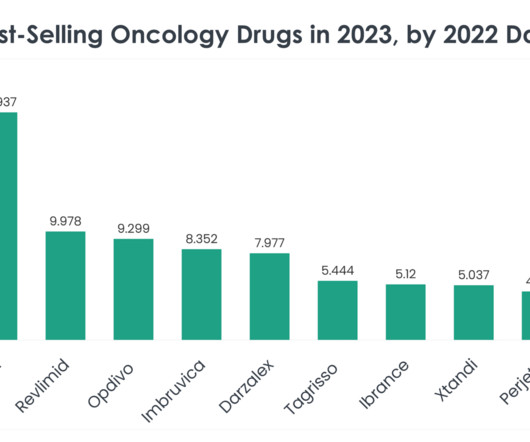
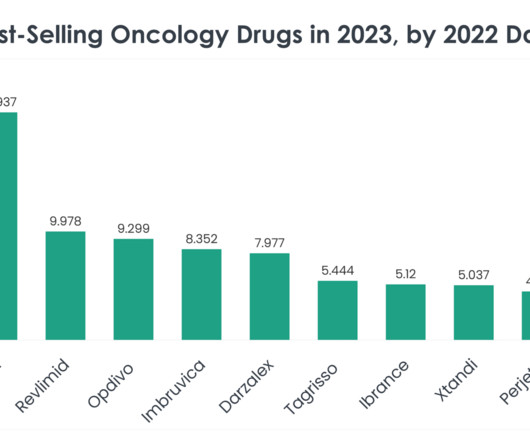
Compounded GLP-1 medications, created by specialized pharmacies under a special US Food and Drug Administration (FDA) allowance due to ongoing shortage, have become increasingly popular for weight loss. The GLP-1 drug market is on its way to becoming one of the most lucrative pharmaceutical markets.























Let's personalize your content