Pfizer’s Ngenla finally secures FDA approval following a rocky road to market
Pharmaceutical Technology
JUNE 28, 2023
Pfizer has announced that the FDA has approved Ngenla (somatrogon-ghla), a paediatric growth hormone deficiency treatment.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
JUNE 28, 2023
Pfizer has announced that the FDA has approved Ngenla (somatrogon-ghla), a paediatric growth hormone deficiency treatment.

BioPharma Reporter
SEPTEMBER 19, 2024
Novartis announced this week that the FDA has approved ribociclib (marketed as Kisqali) for the treatment of people with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR-positive, HER2-negative) stage two or three early breast cancer, who are at high risk of cancer recurrence.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
MAY 16, 2022
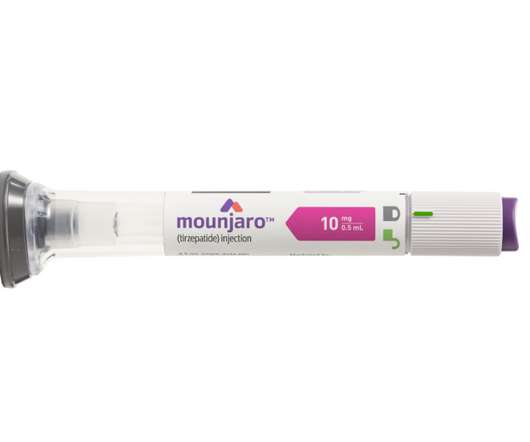
GLP-1 and GIP are hormones involved in blood sugar control and Mounjaro, a first-in-class medicine that activates both GLP-1 and GIP receptors, demonstrated improved blood sugar control. The post Lilly’s new drug Mounjaro (tirzepatide ) wins US FDA approval appeared first on.

Pharmaceutical Technology
SEPTEMBER 13, 2022
Hormone receptor-positive/human epidermal growth factor negative (HR+/HER2-) breast cancer accounts for approximately 70% of all breast cancers, with close to 40,000 new cases diagnosed each year worldwide. The post ESMO 2022: shrinking market for Trodelvy, with fierce ADC competition appeared first on Pharmaceutical Technology.

XTalks
JANUARY 29, 2025
Related: Eli Lillys Zepbound Approved as First Treatment for Obstructive Sleep Apnea The commercial describes Zepbounds mechanism of action as a dual receptor against that works by activating two naturally occurring hormone receptors in [the] body [GLP-1 and GIP-1]. And when it comes to weight loss, change is good, it ends off saying.
Pharmaceutical Technology
JANUARY 22, 2023
In 2022, the FDA approved only 37 new medicines, an underwhelming number compared to 98 in 2018. However, while only around 34% of the approvals in 2018 were for orphan drugs, 54% new approvals in 2022 were for drugs to treat rare diseases. T3 is a major hormone in the blood that regulates thyroid levels.

The Pharma Data
JUNE 28, 2023
FDA Approves Pfizer’s NGENLA™, a Long-Acting Once-Weekly Treatment for Pediatric Growth Hormone Deficiency NEW YORK & MIAMI–(BUSINESS WIRE)– Pfizer Inc. The approval of NGENLA will be significant for children with growth hormone deficiency in the U.S. NYSE: PFE) and OPKO Health Inc.
XTalks
JULY 26, 2024
Norethindrone acetate and ethinyl estradiol are legacy drugs that have been approved since 1968 in a swallowable tablet form for the prevention of pregnancy. The tablet consists of two hormones: norethindrone acetate, a type of progestin, and ethinyl estradiol, a synthetic form of estrogen. percent from 2024 to 2030.

XTalks
JANUARY 30, 2025
Novartis has spent three decades advancing breast cancer treatments, focusing on hormone receptor-positive (HR+), HER2-negative breast cancer, the most common subtype. Last year, the FDA approved Kisqali (ribociclib) to reduce the risk of recurrence in HR+/HER2- early breast cancer.

XTalks
SEPTEMBER 13, 2024
This blog explores the top 10 largest publicly traded healthcare companies by market capitalization , making strides in healthcare delivery and innovation. UnitedHealth Group (UNH) Market Capitalization : $738.67 Elevance Health (ELV) Market Capitalization : $170.91 HCA Healthcare (HCA) Market Capitalization : $136.05

XTalks
MARCH 18, 2025
Incytes recent announcement on the topline results from two Phase III clinical trials of povorcitinib in patients with hidradenitis suppurativa has stirred both hope and caution in the market. Janus kinase one (JAK1) plays a central role in the signaling pathways of several inflammatory cytokines.

pharmaphorum
SEPTEMBER 15, 2021
If approved, linzagolix will be the only drug in the class with a dosing regimen intended for women with uterine fibroids who cannot or do not want to take hormone therapy, as well as options for those women happy to do so, according to ObsEva.

XTalks
MAY 17, 2023
Astellas Pharma recently announced the US Food and Drug Administration (FDA) approval of their new medication, Veozah (fezolinetant) for the treatment of moderate to severe hot flashes and night sweats due to menopause. Hormone replacement therapy is thought to be one of the most effective treatments for vasomotor symptoms.

pharmaphorum
FEBRUARY 1, 2021
Novartis’ multiple sclerosis drug has been given the green light by the European Medicines Agency’s CHMP scientific committee, paving the way for a likely approval in the coming weeks.
XTalks
JANUARY 16, 2025
It is a complication of Graves disease, an immune system condition that causes the thyroid gland to make excess amounts of thyroid hormone. It will rival Amgens Tepezza (teprotumumab-trbw), which became the first approved treatment for thyroid eye disease with its FDA approval in 2020.

Pharmaceutical Technology
OCTOBER 13, 2022
The drug won its original FDA approval in May last year to treat heavy menstrual bleeding that accompanies uterine fibroids in premenopausal women. Myfembree is a combination therapy that includes relugolix, estradiol and norethindrone acetate.

pharmaphorum
APRIL 1, 2022
market is just beginning to heat up. And already in the first quarter of 2022, the FDA approved a third filgrastim biosimilar, Releuko. With 34 approved biosimilars and dozens more in the pipeline, what does this year have in store? Innovator products working to maintain market share.

XTalks
APRIL 29, 2022
Tirzepatide is a novel investigational obesity treatment that contains mimetics of two hormones that are involved in regulating appetite in a single peptide: a GIP (glucose-dependent insulinotropic polypeptide) receptor agonist and a GLP-1 (glucagon-like peptide-1) receptor agonist. percent and 22.5 percent, respectively, compared to placebo.

XTalks
JULY 19, 2024
Compounded GLP-1 medications, created by specialized pharmacies under a special US Food and Drug Administration (FDA) allowance due to ongoing shortage, have become increasingly popular for weight loss. The GLP-1 drug market is on its way to becoming one of the most lucrative pharmaceutical markets.

XTalks
NOVEMBER 3, 2022
Demand for Lilly’s GIP/GLP-1 receptor agonist Mounjaro is also rising because of high patient demand since the drug’s May 13 FDA approval and expanding insurance coverage. In its first quarter on the market since its approval, sales totalled $97 million between July and September in the US.

pharmaphorum
OCTOBER 4, 2022
AstraZeneca and Merck & Co’s Lynparza has been the undisputed market leader in the PARP inhibitor category for some years, but would-be competitors continue to chip away at its market share. The post PARP rivals close in on AZ and Merck’s market leading Lynparza appeared first on.

XTalks
SEPTEMBER 6, 2024
For the international market, Novartis will continue to partner with external isotope suppliers. Lutathera is used to treat adults and children aged 12 years and older with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) that are positive for the hormone receptor somatostatin, including GEP-NETs in the foregut, midgut and hindgut.
XTalks
NOVEMBER 10, 2021
Related: GSK’s Dostarlimab Wins FDA Approval for dMMR Endometrial Cancer. Darbepoetin alfa is a synthetic version of the hormone erythropoietin, which is made in the kidneys and stimulates the production of red blood cells (RBC), or erythrocytes. Market Size and Regulatory Pathway. What is Daprodustat?

XTalks
AUGUST 22, 2024
Yorvipath, developed using Ascendis Pharma’s TransCon technology, is the first and only approved treatment for hypoparathyroidism, marking this approval as a major milestone. Hypoparathyroidism arises when the parathyroid glands fail to produce enough parathyroid hormone (PTH) or when the PTH produced is ineffective.

pharmaphorum
OCTOBER 11, 2020
When added to standard treatment with hormone-based drugs, Ibrance (palbociclib) was unable to achieve better iDFS than hormone therapy alone in women with hormone receptor-positive (HR+), human epidermal growth factor-negative (HER2-) early-stage breast cancer.

XTalks
FEBRUARY 21, 2024
Recently, Eli Lilly revealed promising results from a mid-stage trial, indicating that its popular drug, tirzepatide (marketed as Zepbound and Mounjaro for weight loss and diabetes, respectively), may be an effective treatment for the fatty liver disease metabolic dysfunction-associated steatohepatitis (MASH).
XTalks
OCTOBER 11, 2023
Rybelsus is Novo’s third semaglutide product on the market, a tablet form of the drug used for the treatment of type 2 diabetes. They mimic the action of GLP-1, a hormone that helps regulate blood sugar levels by enhancing insulin secretion.

XTalks
SEPTEMBER 17, 2020
Symptoms and Etiology: Caused by an overproduction of growth hormone, acromegaly primarily affects adults. By reducing the amount of growth hormone in the blood, progressive enlargement of bones should be slowed. By reducing the amount of growth hormone in the blood, progressive enlargement of bones should be slowed.

Delveinsight
DECEMBER 7, 2020
Banyan Biomarkers is one company to be able to market a blood-based diagnostic test in the US market, as it utilizes tech to aid the detection of traumatic brain injuries and concussions. In February 2018, the San Diego-based company was granted a de novo request from FDA for the Banyan Brain Trauma Indicator.

XTalks
MARCH 8, 2024
The biosimilar injections, Jubbonti (denosumab-bddz) and Wyost (denosumab-bddz), are approved as interchangeable biosimilars to Prolia and Xgeva, respectively. Despite the approvals, the market launches of Jubbonti and Wyost are uncertain due to ongoing patent litigation between Sandoz and Amgen.

The Pharma Data
DECEMBER 16, 2020
Food and Drug Administration (FDA) approval and the launch of Aminocaproic Acid Tablets USP, 500mg. market for this product is approximately $12.7 “This is ANI’s seventh generic product launch in 2020 and reaffirms our commitment to increasing the pace of market introductions for our products.

XTalks
MARCH 15, 2023
FDA-approved ADHD-specific non-stimulants are a relatively newer class of medication designed to treat ADHD that have much fewer side effects than stimulants. Norepinephrine is a hormone and neurotransmitter that helps in alertness and maintaining control in high-stress situations. In 2017, Strattera’s total revenue was $618.2

Pharmaceutical Technology
AUGUST 8, 2022
According to the trial data, Enhertu lowered disease progression or mortality risk by 50% compared with chemotherapy according to the physician’s choice in HER2-low metastatic breast cancer patients with hormone receptor (HR)-positive disease or HR-negative disease. months compared with 5.1 months for the chemotherapy arm.

XTalks
JULY 6, 2023
Pfizer has been going strong on the approval front, having secured its fifth US Food and Drug Administration (FDA) approval in the past five weeks, with the most recent being for the company’s once-weekly human growth hormone analog Ngenla (somatrogon-ghla).

Pharmaceutical Technology
MAY 22, 2023
As supplies for Besins Healthcare’s hormone replacement therapy (HRT) medication Utrogestan (progesterone) experience shortages in the UK, the Department of Health and Social Care has issued a Serious Shortage Protocol (SSP) addressing the measures being made to mitigate the issue. Please check your email to download the Report.
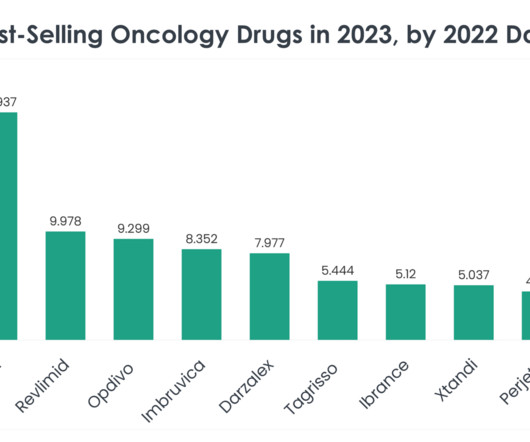
XTalks
FEBRUARY 5, 2024
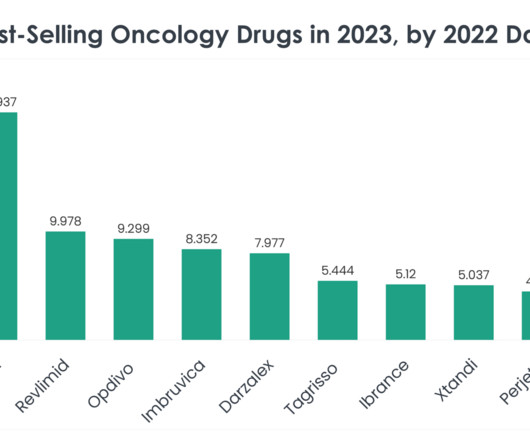
As we delve into the specifics of these best-selling oncology drugs, we uncover the underlying factors contributing to their market dominance, from clinical efficacy and safety profiles to strategic market positioning and patient accessibility. Price of Revlimid: A supply of 28 oral 2.5 mg capsules is $24,576.

XTalks
FEBRUARY 19, 2021
Evofem Biosciences announced the launch of their new national campaign called, “Get Phexxi,” designed to raise awareness about the company’s non-hormonal birth control method. In May 2020, the US Food and Drug Administration (FDA) approved Phexxi , a non-hormonal contraceptive for women.

XTalks
NOVEMBER 9, 2022
Glucagon-like peptide-1 (GLP-1) receptor agonists are a newer class of diabetes drugs that have the potential to double as weight loss drugs, widening their lucrative market potentials. Novo’s obesity version of semaglutide (at a higher dose) is marketed as Wegovy and was approved in 2021. billion in 2021 to $61.6

pharmaphorum
DECEMBER 23, 2020
2020 also saw some of the first “tumour agnostic” cancer drugs get to market, with Bayer’s Vitravki (larotrectinib) getting funding in the UK for tumours with confirmed neurotrophic tyrosine receptor kinase (NTRK) gene fusions.”.
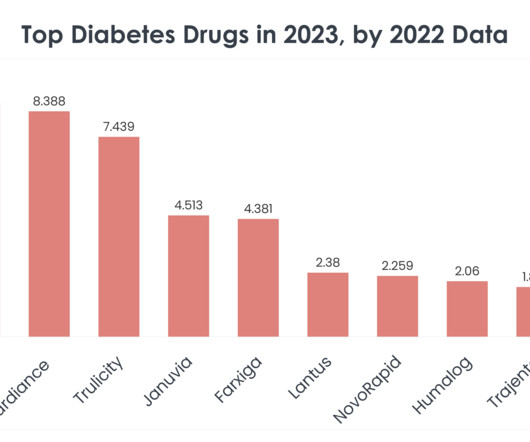
XTalks
FEBRUARY 8, 2024
Understanding the market dynamics of diabetes treatments becomes crucial for professionals across these industries. Ozempic (Semaglutide) Ozempic sales in 2022: $8.713 billion Company/Developer: Novo Nordisk Date of first FDA approval: December 5, 2017 Indications Ozempic is FDA-approved for: Type 2 diabetes Price of Ozempic: $1,029 for 1.5
XTalks
FEBRUARY 5, 2024
As we delve into the specifics of these best-selling oncology drugs, we uncover the underlying factors contributing to their market dominance, from clinical efficacy and safety profiles to strategic market positioning and patient accessibility. Price of Revlimid: A supply of 28 oral 2.5 mg capsules is $24,576.

Intouch Solutions
AUGUST 5, 2021
This then leads many of those same people to online forums like the TransDIY subreddit that’s dedicated to do-it-yourself hormone replacement therapy, or DIY HRT. DIY HRT is a combination of non-prescribed, often unregulated, hormone medicines and self-performed surgeries that are administered without the supervision of an HCP.

Delveinsight
AUGUST 2, 2020
The dynamics of the cancer cachexia market is expected to gain momentum as several companies are testing the waters, advancing cancer cachexia pipeline. Cachexia is observed as a result of underlying diseases, including cancer, AIDS, tuberculosis, chronic heart failure, hormonal deficiency, and others.

The Pharma Data
OCTOBER 14, 2020
WAKIX is the first and only treatment approved by the FDA for people with excessive daytime sleepiness or cataplexy associated with narcolepsy that is not scheduled as a controlled substance by the U.S. WAKIX received FDA approval for the treatment of excessive daytime sleepiness in adult patients with narcolepsy in August 2019.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content