GSK pays $90M to gain access to Scynexis antifungal
Bio Pharma Dive
MARCH 30, 2023
The licensing deal for the FDA-approved medicine, Brexafemme, includes milestone payments that could add up to $503 million.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
MARCH 30, 2023
The licensing deal for the FDA-approved medicine, Brexafemme, includes milestone payments that could add up to $503 million.
Pharmaceutical Technology
OCTOBER 7, 2022
The US Food and Drug Administration (FDA) is presently reviewing teplizumab for the delay of clinical T1D in people who are at risk. A decision on the approval of the Biologics License Application (BLA) is anticipated on 17 November. An anti-CD3 monoclonal antibody, teplizumab is in the developmental stage to delay T1D.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
APRIL 6, 2023
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted ADvantage Therapeutics’ immunotherapy AD04 an Innovation Passport for the treatment of Alzheimer’s disease. A recent wave of monoclonal antibodies, including the FDA-approved Eisai / Biogen ’s Leqembi (lecanemab), is expected to shake up the space.

pharmaphorum
NOVEMBER 24, 2020
The FDA has approved a new use for Xofluza (baloxavir marboxil) from Roche’s Genentech unit, to prevent people developing flu after coming into contact with an infectious person. Xofluza has already been on the market for two years, and already had licensed uses to treat uncomplicated flu and those at high risk of complications.

XTalks
APRIL 3, 2024
Related: Novartis’ Fabhalta Gets FDA Approval for Rare Complement Blood Disorder The FDA’s green light for Voydeya comes three months after the Japanese Ministry of Health, Labour and Welfare (JHLW) became the first regulator in the world to back the therapy. AstraZeneca is also venturing into cell therapy and genetic medicine.

XTalks
FEBRUARY 4, 2025
The company plans to use these funds to advance its groundbreaking research in small molecule precision medicines, targeting renal, cardiovascular and metabolic diseases, including obesity. To further expand its research, Maze has entered several strategic partnerships and licensing agreements. The offering, which includes 8.75
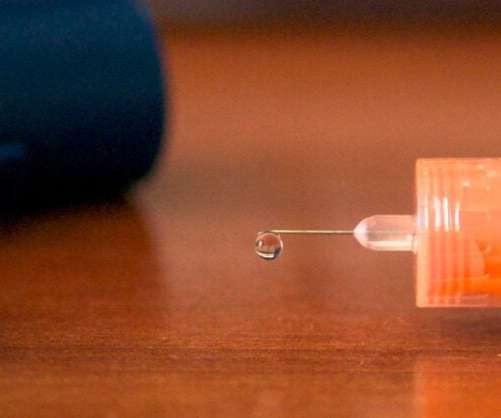
Scienmag
AUGUST 5, 2020
Medical University of South Carolina clinician-innovators targeted the problem of needle-stick injuries during intraoperative monitoring; their invention, a safer needle, is now FDA approved and licensed to Rhythmlink for rollout this fall in hospitals Credit: Photograph by Joshua Aaron Photography, provided by Rhythmlink.

Pharmaceutical Technology
NOVEMBER 1, 2022
The European Commission (EC) has granted orphan medicinal product designation for Karyopharm Therapeutics and the Menarini Group’s Nexpovio (selinexor) to treat myelofibrosis (MF). The companies signed an exclusive licensing agreement in December 2021. The therapy is commercialised in the US as Xpovio.

Camargo
NOVEMBER 11, 2020
Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. When the FDA requires a product’s labeling to include a boxed warning (also called a “black box warning” because the text is surrounded by black border), the potential market value of the drug often drops severely.
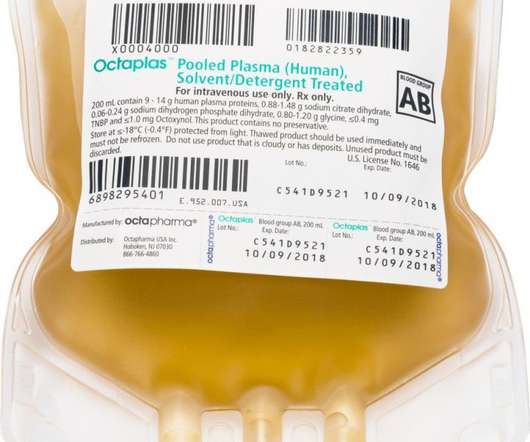
Scienmag
MARCH 28, 2021
Octaplas™ and fibryga® receive new product labeling following FDA’s approval of BLA supplements to update therapy research; FDA expands fibryga® indication to include treatment of children under 12 years of age Credit: Octapharma USA PARAMUS, N.J. March 29, 2021) – The U.S.

XTalks
DECEMBER 18, 2024
Trial data for both the pediatric and adult trials were published in The New England Journal of Medicine. Crenessity marks the third approved drug for Neurocrine. In 2018, the company also received approval for Orilissa (elagolix), which it licenses to AbbVie, for the treatment of endometriosis.

Pharmaceutical Technology
MARCH 27, 2023
Iovance Bioterapeutics announced it has completed its rolling Biologics License Application (BLA) submission to the U.S. Food and Drug Administration (FDA) for Lifileucel. Marc Hurlbert, CEO of the Melanoma Research Alliance (MRA) said he hopes for a quick FDA approval.

Pfizer
JULY 8, 2022
FDA Approval of their COVID-19 Vaccine COMIRNATY® For Adolescents 12 through 15 Years of Age. FDA Approval of their COVID-19 Vaccine COMIRNATY® For Adolescents 12 through 15 Years of Age. Pfizer and BioNTech also filed these data with the European Medicines Agency (EMA) and other regulatory authorities around the world.
Pharmaceutical Technology
FEBRUARY 26, 2023
In November 2022, Ferring Pharmaceuticals’s Rebyota became the first FDA-approved microbiome-based therapeutic to treat recurrent CDI. But the approval came through after an FDA Advisory Committee meeting. While Rebyota was the first to be approved, more could be coming up soon, says Gilbert.

Pharmaceutical Technology
MARCH 31, 2023
Brexafemme is a novel, approved anti-fungal medicine with a broad spectrum of activity against existing and emerging resistant strains of fungi. It will also receive an additional $15m in milestone payments, upon receipt of US FDA approval for an additional indication. The company will receive up to $245.5m

Pharmaceutical Technology
NOVEMBER 9, 2022
An approval decision on the gene therapy, also known as EtranaDez, is expected by the end of this month. CSL Behring has a commercialisation and license agreement to develop EtranaDez. After its approval, bluebird had set Zynteglo’s price at roughly EUR1.6 million ($1.8 million at the time). million price tag.

pharmaphorum
NOVEMBER 24, 2020
Alnylam’s Givlaari, FDA-approved a year ago for acute hepatic porphyria, costs $575,000 per year at full price in the US, although after discounts the figure is more likely to be in the region of $442,000 per year. Alnylam licensed inclisiran to The Medicines Company, which was bought last year by Novartis for $9.7

Pharmaceutical Technology
FEBRUARY 17, 2023
In January, amidst calls to improve patient safety by optimizing licensed drug formulations, the FDA released a draft guidance that signalled a departure from the most commonly used method of identifying a new therapy’s ideal dosage. The focus on identifying the ideal drug dosage is not new.
Pharmaceutical Technology
AUGUST 23, 2022
The companies have also commenced rolling submission for conditional marketing authorization from the European Medicines Agency (EMA) for this Omicron BA.4/BA.5-adapted 5-adapted bivalent vaccine.

Pfizer
JULY 19, 2022
Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.1 Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.1 a second booster dose to individuals 50 years of age and older who have received a first booster dose of any authorized or approved COVID-19 vaccine.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older.

pharmaphorum
SEPTEMBER 29, 2021
Amazon is advancing into healthcare on multiple fronts, launching its own pharmacy business for medicines delivery in the US last year, whilst also entering the health wearables market with its own Halo device, and bolstering the health-related capabilities of its Alexa virtual assistant.

pharmaphorum
SEPTEMBER 15, 2021
If approved, linzagolix will be the only drug in the class with a dosing regimen intended for women with uterine fibroids who cannot or do not want to take hormone therapy, as well as options for those women happy to do so, according to ObsEva.

Pharmaceutical Technology
SEPTEMBER 2, 2022
The rate of drug approvals could be on the rise. In a recent report, GlobalData revealed that the US Food and Drug Administration (FDA) approved 122 new drug applications (NDAs) and biologic license applications (BLAs) in 2021. This figure represents a 2.4% increase over the 2016–2020 period average.

Camargo
DECEMBER 13, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. The regulations regarding BLAs for therapeutic biological products are included in 21 CFR parts 600 , 601 , and 610.

pharmaphorum
JANUARY 11, 2023
The partnership will build on Anima’s previous discovery and development collaborations with Eli Lilly in July 2018 and with Takeda in 2021, giving AbbVie exclusive rights to license and further develop and commercialise the programmes. Anima’s wholly owned pipeline programmes, meanwhile, are in fibrosis, oncology, and neuroscience.

pharmaphorum
FEBRUARY 1, 2021
Novartis’ multiple sclerosis drug has been given the green light by the European Medicines Agency’s CHMP scientific committee, paving the way for a likely approval in the coming weeks.

pharmaphorum
OCTOBER 3, 2022
Translarna was the first licensed treatment for DMD which addresses the loss of dystrophin. Translarna has had European approval for usage since 2014 and has also been available under terms similar to the MAA to DMD patients in Scotland since 2021. The post Translarna not approved by NICE for DMD on NHS appeared first on.

Pharmacy Checkers
JANUARY 10, 2020
This post is mostly a story about a very well manufactured, safe and effective, foreign version of an FDA-approved drug. These drugs are normally far less expensive than the FDA-approved version sold in the U.S. The exact same FDA-approved drugs are sold in America and other countries, but with different labels.
The Pharma Data
AUGUST 19, 2021
today announced that the company’s Marketing Authorization Application (MAA) for lenacapavir, an investigational, long-acting HIV-1 capsid inhibitor, has been fully validated and is now under evaluation with the European Medicines Agency (EMA). Additional data from the CAPELLA study will be presented at a future scientific conference.
XTalks
AUGUST 21, 2023
The FDA also issued a Rare Pediatric Disease Priority Review Voucher (PRV) to Ipsen that can be used for subsequent drug applications that would not qualify for a priority review. The FDA approval of Sohonos is a breakthrough for the US FOP community,” said Howard Mayer, head of R&D at Ipsen, in the company’s news release.

pharmaphorum
DECEMBER 23, 2021
At its second attempt, Novartis has won FDA approval for its cholesterol lowering drug inclisiran, which can reduce levels with just two injections a year. Novartis added inclisiran to its pipeline after buying The Medicines Company – which had licensed the drug from Alnylam – for $9.7 It has a long way to go.

XTalks
APRIL 17, 2023
These results are very promising for patients with atopic dermatitis,” said Jonathan Silverberg, MD, professor of dermatology at George Washington University School of Medicine & Health Sciences and co-investigator of the studies, in the company’s press release. Dupixent inhibits signaling of both IL-4 and IL-13.

XTalks
JULY 19, 2024
Compounded GLP-1 medications, created by specialized pharmacies under a special US Food and Drug Administration (FDA) allowance due to ongoing shortage, have become increasingly popular for weight loss. Moreover, 15 percent of flagged ads offer the medication without the need of a prescription.
Triage Cancer
AUGUST 18, 2020
Steven Joffe from the University of Pennsylvania’s Perelman School of Medicine found that the FDA approved 99% of all expanded access requests for almost 9,000 investigational drugs. To apply for Expanded Access: The patient consults with their licensed physician to make sure they have exhausted all other treatment options.
pharmaphorum
SEPTEMBER 26, 2022
Astellas has won a reprieve in its attempt to stop Pfizer’s generic medicines unit Hospira launching a copycat version of its pharmacologic stress agent Lexiscan in the US – but only for a couple of weeks. AmphaStar and Glenmark Pharma also claimed FDA approval for versions of the drug earlier this year.
pharmaphorum
MARCH 16, 2022
Viatris (formerly Mylan) has become the first drugmaker to win full FDA approval for a generic version of AstraZeneca’s top-selling respiratory drug Symbicort, although launch plans remain uncertain for now. The post Update: FDA clears first generic of AZ’s blockbuster Symbicort appeared first on.

pharmaphorum
APRIL 27, 2021
Since then, Merck KGaA and Pfizer have picked up a full FDA approval for their immunotherapy Bavencio (avelumab) as a first-line maintenance therapy for this type of cancer, and that could potentially raise the level of evidence needed to keep Keytruda and Tecentriq available to patients.

The Pharma Data
DECEMBER 17, 2020
NASDAQ: REGN ) today announced that the New England Journal of Medicine (NEJM) has published initial clinical data from an ongoing seamless Phase 1/2/3 trial of the antibody cocktail casirivimab and imdevimab in non-hospitalized patients with COVID-19. Casirivimab and imdevimab injection is not FDA approved for any use.

The Pharma Data
NOVEMBER 30, 2020
1, 2020 /PRNewswire/ — Sosei Group Corporation (“the Company”) (TSE: 4565) announces it has entered into a global collaboration and license agreement with Biohaven Pharmaceutical Holding Company Ltd. (“Biohaven”, NYSE: BHVN). . TOKYO and CAMBRIDGE, England , Dec.
pharmaphorum
JANUARY 4, 2023
It looks like Astellas has been unsuccessful in its attempt to block Pfizer’s generic medicines unit Hospira from launching a copycat version of big-selling pharmacologic stress agent Lexiscan in the US. AmphaStar and Glenmark Pharma also claimed FDA approval for versions of the drug earlier this year.
The Pharma Data
APRIL 14, 2021
Food and Drug Administration (FDA) has approved the company’s supplemental Biologics License Application for Xolair® (omalizumab) prefilled syringe for self-injection across all approved U.S. with Xolair since its initial approval in 2003. The Roche Group’s immunology medicines include: Actemra ® /RoActemra ® ?(tocilizumab)

XTalks
FEBRUARY 29, 2024
After its accelerated approval in January 2023, Leqembi received full US Food and Drug Administration (FDA) approval in July 2023. The journey of Aduhelm from its accelerated approval and predicted blockbuster status to discontinuation has been fraught with controversy, debate and ultimately, disappointment.

pharmaphorum
FEBRUARY 15, 2022
Mirvetuximab soravtansine showed efficacy in a phase 3 trial in ovarian cancer patients last November and is due to be filed for FDA approval in the coming weeks. Lilly is thought to be the first pharma company to sign up to use the new camptothecin payload platform.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content