FDA Approves First Eylea Interchangeable Biosimilars Yesafili and Opuviz
XTalks
JUNE 3, 2024
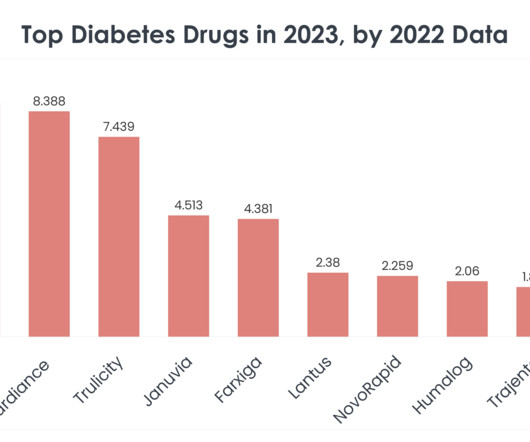
Yesafili and Opuviz are approved to treat the same conditions as Regeneron’s vascular endothelial growth factor (VEGF) inhibitor Eylea — neovascular (wet) age-related macular degeneration (AMD), diabetic retinopathy, macular edema following retinal vein occlusion (RVO) and diabetic macular edema (DME). In 2022, global Eylea sales hit $9.6




















Let's personalize your content