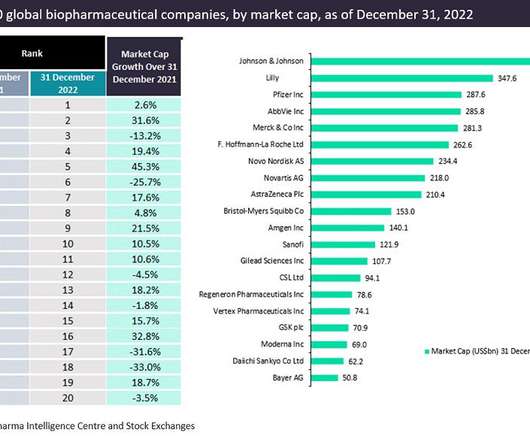
Market cap growth surges for over half of the top biopharmaceutical companies in 2022
Pharmaceutical Technology
FEBRUARY 8, 2023
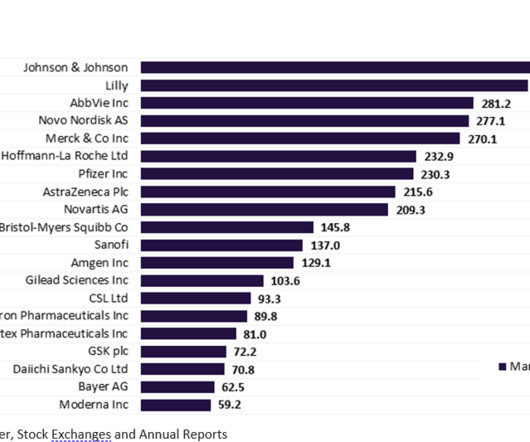
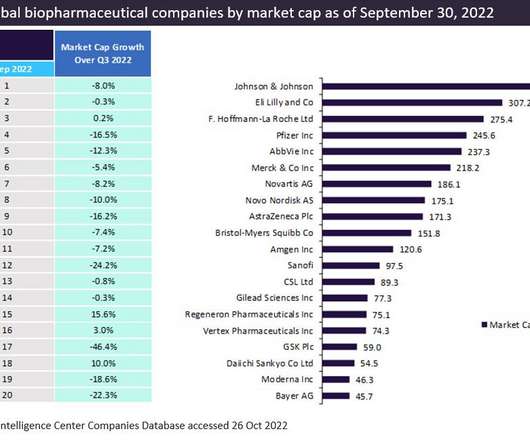
The top 20 companies in the global biopharma industry saw 5.4%* aggregate market capitalization from $3.43 Pfizer maintained its third-place position in the market in 2022, reporting a market capitalization of $287.6 witnessed the biggest market capitalization growth of 45.3% trillion to $3.61 billion on Dec 31, 2022.








































Let's personalize your content