How tech-driven hubs in specialty pharmacy can improve the patient experience
pharmaphorum
JULY 29, 2021
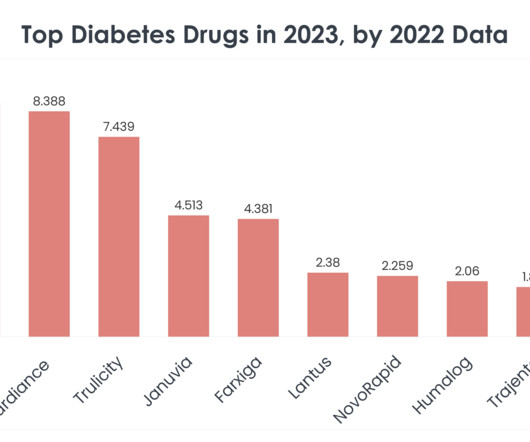
It’s estimated specialty medications account for 75% of the approximately 7,000 prescription drugs currently in development, and by 2022, more than 60% of the 600 drugs expected to gain FDA approval will be specialty medications. The problem is, many specialty pharmacies don’t currently have the right tools to support their patients.












































Let's personalize your content