FDA approves Amneal and Shilpa’s oncology product BORUZU
Pharmaceutical Technology
SEPTEMBER 6, 2024
The US FDA has approved Amneal Pharmaceuticals and Shilpa Medicare’s oncology product BORUZU for subcutaneous administration.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmaceutical Technology
SEPTEMBER 6, 2024
The US FDA has approved Amneal Pharmaceuticals and Shilpa Medicare’s oncology product BORUZU for subcutaneous administration.
XTalks
FEBRUARY 5, 2025
Celltrions Actemra (tocilizumab) biosimilar Avtozma (tocilizumab-anoh) has received FDA approval for multiple indications, including rheumatoid arthritis (RA), giant cell arteritis (GCA), polyarticular juvenile idiopathic arthritis (pJIA), systemic juvenile idiopathic arthritis (sJIA) as well as COVID-19.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
SEPTEMBER 17, 2022
The therapy, called Skysona and cleared to treat cerebral adrenoleukodystrophy, is the product of more than a decade of work by Bluebird. It will cost $3 million.

Pharmaceutical Technology
DECEMBER 1, 2022
The US Food and Drug Administration (FDA) has granted approval for Ferring Pharmaceuticals’ faecal microbiota product, Rebyota, to prevent Clostridioides difficile infection (CDI) recurrence in people aged 18 years and above. A live biotherapeutic, Rebyota is intended for usage following the completion of antibiotic treatment.
Pharmaceutical Technology
MARCH 8, 2023
It offers an alternative to a product for which there is a shortage. The regulatory approval marks the company’s first product to receive approval in the US market. The post US FDA approves Shorla’s oncology drug for T-cell leukaemia appeared first on Pharmaceutical Technology.

XTalks
NOVEMBER 14, 2024
PTC Therapeutics has gained US Food and Drug Administration (FDA) approval for its new gene therapy, Kebilidi (eladocagene exuparvovec), for treating aromatic L-amino acid decarboxylase (AADC) deficiency.

Bio Pharma Dive
JULY 29, 2021
An injectable insulin from Viatris has become the first-ever biosimilar product that can be directly substituted for a marketed biologic, a long-awaited decision that could put pricing pressure on other diabetes drugs.

XTalks
DECEMBER 19, 2024
It includes Roches Alecensa (alectinib), Takedas Alunbrig (brigatinib) and Pfizers third-generation Lorbrena (lorlatinib), which have all surpassed Xalkori in performance, leading to their respective FDA approvals for first-line treatment. billion Chinese yuan ($321 million), derived from five products, including ensartinib in China.

Bio Pharma Dive
NOVEMBER 16, 2023
Acquired via a $4 billion biotech buyout, Augtyro is one of an array of new products the pharma hopes will offset patent expirations for current top sellers.

XTalks
NOVEMBER 7, 2024
Emrosi consists of minocycline, a tetracycline antibiotic, to target bacterial infection that can underlie rosacea. Research suggests that certain bacteria, particularly those associated with Demodex mites (tiny mites that naturally live on human skin) like Bacillus oleronius , could contribute to rosacea in some individuals.
XTalks
APRIL 1, 2025
XTALKS WEBINAR: Keys to Success in Clinical Trials: A Strategic Guide for Biotechs and Startups Live and On-Demand: Thursday, May 22, 2025 , at 11am EDT (5pm CEST / EU-Central) Register for this free webinar to learn how biotechs can navigate endpoint challenges in clinical trials and accelerate their path to drug approval. billion ($1.4

Pharmaceutical Technology
JANUARY 8, 2024
Novartis has received the US FDA approval to commercially manufacture Pluvicto at its new RLT manufacturing facility.

Pharmaceutical Technology
JUNE 21, 2023
AGEPHA Pharma managing director Antonia Riel-Köllmann stated: “As the third generation of my family dedicated to developing high-quality European pharmaceuticals, it’s a privilege to bring this life-sustaining therapy, which represents the company’s first product launch in the US, to the global market. “We

Pharmaceutical Technology
JANUARY 16, 2023
It is claimed to be the first advanced therapy from the company’s central nervous system (CNS) product portfolio for marketing in the US. Rykindo is our first new drug developed in-house and approved for marketing in the US, demonstrating our long-standing commitment to serving patients around the world with innovative therapies. “At

Pharmaceutical Technology
MAY 4, 2023
The US Food and Drug Administration (FDA) approved Kamada’s application to manufacture Cytogam (cytomegalovirus immune globulin intravenous [human]) at its facility located in Beit Kama, Israel. The regulatory approval represents the completion of the Cytogam technology transfer process from CSL Behring, its previous manufacturer.

Pharmaceutical Technology
JUNE 9, 2023
The US Food and Drug Administration (FDA) has granted approval to commence commercial production at Bristol Myers Squibb’s new advanced cell therapy manufacturing facility in Devens, Massachusetts. Today’s approval underscores our commitment to deliver our transformational CAR T cell therapies to more patients.”

Bio Pharma Dive
SEPTEMBER 9, 2020
Results from two Phase 3 studies put Merck in position to ask for FDA approval of its shot later this year. Pfizer may not be far behind with a rival product.

Pharmaceutical Technology
JUNE 12, 2023
It noted that the injection’s latest approval will help reduce the recent supply issues for the product in the country. This latest move marks Milla Pharmaceuticals’ second ‘first cycle’ FDA approval for an abbreviated new drug application (ANDA) and second paragraph IV filing.
XTalks
JANUARY 21, 2025
TriClip G4 System Manufacturer/developer : Abbott Medical Date of FDA approval : April 1, 2024 Approved for : Tricuspid regurgitation (TR). Date of FDA approval : March 29, 2024 Approved for : To detect exposure to human parvovirus B19. TriClip size comparison photo. Photo courtesy of Abbott Medical.

Pharmaceutical Technology
MARCH 27, 2023
It is claimed to be the first and only therapy to receive approval in the US for the treatment of APDS, a rare and progressive primary immunodeficiency. After assessing the New Drug Application (NDA) under priority review, the FDA granted the approval based on the data obtained from a Phase II/III clinical trial.

CTTI (Clinical Trials Transformation Initiative)
MARCH 19, 2025
A new publication from the FDA Oncology Center of Excellence (OCE) and the Clinical Trials Transformation Initiative (CTTI), published in the Clinical Cancer Research journal, provides important insights into how decentralized clinical trial (DCT) elements were used in cancer trials leading to FDA approval during the COVID-19 pandemic.

FDA Law Blog
NOVEMBER 10, 2024
Food and Drug Administration (FDA) plays a pivotal role in fostering the development of treatments for rare diseases through its Orphan Products Grants Program. Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field.

Pharmaceutical Technology
DECEMBER 14, 2022
Harrow has signed a binding agreement to acquire exclusive US commercial rights to five ophthalmic products of Novartis. These products, namely, Ilevro, Vigamox, Maxidex, Nevanac and Triesence, have received approval from the Food and Drug Administration (FDA).
Pharmaceutical Technology
MAY 25, 2023
Yuflyma represents the company’s fifth biosimilar and second anti-TNF biosimilar to receive US FDA approval. The regulatory approval was based on a comprehensive data package of preclinical, analytical and clinical trials. It will be offered to patients in prefilled syringe and autoinjector administration options.

pharmaphorum
JANUARY 26, 2022
Immunocore has secured a piece of biotech industry history, becoming the first company to get an FDA approval for a cancer therapeutic based on T cell receptor (TCR) technology. It will be the UK biotech’s first commercial product, coming more than 14 years after it spun out of the UK subsidiary of Germany’s Medigene.

XTalks
NOVEMBER 28, 2023
This liquid formulation of metronidazole is the sole FDA-approved liquid option, offering a groundbreaking prescribing alternative for patients encountering difficulties in swallowing or facing taste-related obstacles. With a 24-month shelf life and no need for refrigeration, Likmez provides a convenient option for patients.
Pharmaceutical Technology
JUNE 13, 2023
The US Food and Drug Administration (FDA) has approved Neobiosis’ investigational new drug (IND) application for ViXome to treat post-Covid-19 syndrome (also known as long Covid). ViXome is an acellular product derived from amniotic fluid.
Pharmaceutical Technology
MARCH 1, 2023
In the last few years, there have been growing calls to onshore the manufacturing of certain pharma products, including active pharmaceutical ingredients (API), to ensure a smooth supply and to minimise shortages in the European Union (EU). You can also subscribe here to receive email notifications when a new issue is available.

XTalks
AUGUST 12, 2024
Related: Asthma Drug Xolair Wins FDA Approval as First Treatment for Multiple Food Allergies “Today’s approval provides the first epinephrine product for the treatment of anaphylaxis that is not administered by injection. If you want your company to be featured on Xtalks.com, please email ayeshar@xtalks.com.

Pharmaceutical Technology
AUGUST 5, 2022
Thus, the biosimilar presented a similar efficacy to the reference product. All in all, following rigorous testing procedures, results for the safety and efficacy of Cimerli were highly promising, meeting the FDA’s meticulous standards, which ultimately led to its approval and prospective launch in October this year.
XTalks
JANUARY 22, 2025
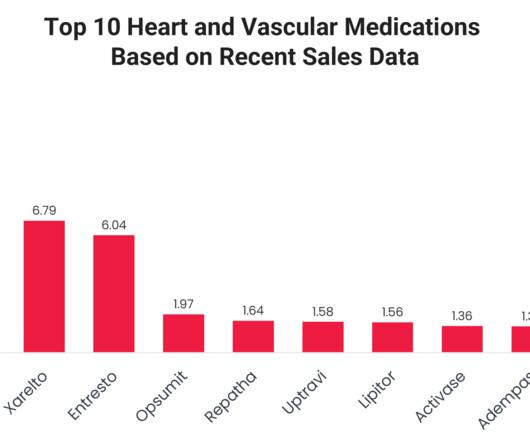
Their widespread adoption is driven by strong clinical evidence, broad FDA-approved indications and continued advancements in treatment protocols. Although the FDA approved generic versions from Micro Labs Limited and Mylan Pharmaceuticals Inc. Eliquis (Apixaban) Eliquis 2023 sales :$12.21

XTalks
JANUARY 2, 2024
Filsuvez topical gel is a sterile botanical drug product designed for topical use, containing birch triterpenes within an oil base. For instance, Vyjuvek , the first FDA-approved gene therapy for DEB, is priced at $24,250 per vial. Regarding the price of Filsuvez, it has not yet been publicly disclosed by the company.

pharmaphorum
DECEMBER 30, 2021
Johnson & Johnson’s much-touted crop of bispecific antibodies for cancer generated its first commercial product in May, and the drugmaker has now filed for FDA approval of a second candidate. The post J&J goes after another FDA approval for a cancer bispecific appeared first on.

XTalks
FEBRUARY 15, 2023
Polarean is a medical imaging company focused on improving the state of lung imaging that recently received FDA approval for its drug-device combination product, Xenoview. Tune into the episode to learn more about the FDA approval of Xenoview, including the journey to its approval.
XTalks
NOVEMBER 30, 2023
Pfizer spinout SpringWorks Therapeutics’ Ogsiveo (nirogacestat) has received US Food and Drug Administration (FDA) approval for the treatment of desmoid tumors, an ultra-rare subtype of non-cancerous soft tissue sarcomas that can cause severe pain and disfigurement.

Pharmaceutical Technology
JUNE 15, 2023
By eliminating formulation steps common with other pemetrexed products, we are improving provider efficiencies while reducing the risk of medication errors. The product will be offered in 500mg/50mL,100mg/10mL and 1,000mg/100mL vial sizes.

Camargo
MAY 19, 2021
The Camargo Blog has published a four-part blog series highlighting those designation programs available specifically for products with rare disease indications. The HUD designation program is designed for medical devices and is similar to the Orphan Drug Designation (ODD) program for drugs and biological products.

Pharmaceutical Technology
JUNE 19, 2023
Myelofibrosis is a rare bone marrow cancer that causes the dysfunctional production of blood cells, leading to extensive bone marrow scarring, causing severe anaemia. Novartis and Incyte Corp’s Jakafi was the first FDA-approved drug for the treatment of myelofibrosis in November 2011.
XTalks
AUGUST 7, 2023
has received US Food and Drug Administration (FDA) approval for treating molluscum contagiosum in adult and pediatric patients aged two years and older in the US. Formerly known as VP-102, Ycanth is the first cantharidin formulation approved for this purpose.

Pharmaceutical Technology
MAY 16, 2023
Utilisation of the product for these indications is authenticated by data from one adequate and controlled open-label trial in infants, as well as additional safety data from four trials in 164 paediatric patients and supportive paediatric information from other approved ibuprofen products.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition. Data from two Phase III studies, U-EXCEED and U-EXCEL, involving 857 patients was used to support the approval.

Pharmaceutical Technology
APRIL 17, 2023
If accepted, Rexulti would be the first FDA-approved treatment for AAD. Also known as brexpiprazole, the FDA first approved Rexulti as a treatment for adults with schizophrenia and as an add-on treatment for adults with major depressive disorder in July 2015.

Fierce Pharma
JUNE 20, 2024
The med is the first new chemical entity to win FDA approval specifically to treat primary axillary hyperhidrosis, or excessive underarm sweating. It's also Botanix's first approved product. Everybody sweats, but for the 10 million people in the U.S.

pharmaphorum
NOVEMBER 11, 2024
Autolus gets its first product approval, an FDA green light for anti-CD19 CAR-T Aucatzyl for B-cell acute lymphoblastic leukaemia
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content