FDA approves first-of-its-kind RNA drug for hemophilia
Bio Pharma Dive
MARCH 28, 2025
The Sanofi drug, known as fitusiran and now Qfitlia, was approved on Friday for a broad group of people with the rare bleeding disorder.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
MARCH 28, 2025
The Sanofi drug, known as fitusiran and now Qfitlia, was approved on Friday for a broad group of people with the rare bleeding disorder.

Pharmaceutical Technology
JULY 29, 2022
Last week, CAMP4 Therapeutics announced the close of a $100 million Series B round , which will be used to advance their regulatory RNA (regRNA)-focused programs. According to Bumcrot, regRNAs are “RNAs that arise out of the non-coding genome”. A nitrogen-binding drug, its global sales were $292m in 2021, as per GlobalData.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MARCH 1, 2023
Further, the acceptance of new mRNA vaccines has rejuvenated activity within previously established categories of RNA therapeutics including lifesaving antisense technologies. These include antisense oligonucleotides (ASO), RNA interference (RNAi), and RNA aptamers.
XTalks
JANUARY 21, 2025
TriClip G4 System Manufacturer/developer : Abbott Medical Date of FDA approval : April 1, 2024 Approved for : Tricuspid regurgitation (TR). Date of FDA approval : March 29, 2024 Approved for : To detect exposure to human parvovirus B19. TriClip size comparison photo. Photo courtesy of Abbott Medical.

XTalks
JANUARY 3, 2024
Wainua is the only FDA-approved drug for the treatment of ATTRv-PN that can be self-administered via an auto-injector. Approval of Wainua represents a meaningful advancement in treatment, one that gives those who are living with transthyretin-mediated amyloid polyneuropathy help managing the disease,” said Michael J.
XTalks
JUNE 20, 2022
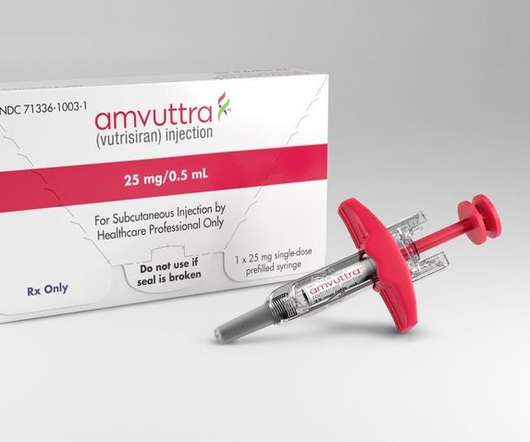
Alnylam Pharmaceuticals, a leading RNA interference (RNAi) therapeutics biopharmaceutical company, announced it received approval from the US Food and Drug Administration (FDA) for its RNAi therapeutic Amvuttra (vutrisiran) for the treatment of the polyneuropathy of hereditary transthyretin-mediated (ATTR) amyloidosis in adults.
XTalks
JULY 14, 2022
Related: Vtama (tapinarof) Cream Gains FDA Approval for the Treatment of Plaque Psoriasis in Adults. The company has partnered with the likes of HemoShear Therapeutics to discover new gout therapies, as well as Arrowhead Pharmaceuticals to develop an RNA interference (RNAi) therapeutic for the disease.

XTalks
AUGUST 18, 2020
An important feature of the test that contributes to its reduced cost is the fact that sample processing does not require a separate nucleic acid (RNA) extraction step. This is significant because shortages of RNA extraction kits have been a recurrent issue since the beginning of the pandemic. And now it’s getting approval.

XTalks
NOVEMBER 15, 2024
XTALKS WEBINAR: Solutions for Vaccine Innovation and Gene Therapy: Unlocking the Power of RNA Live and On-Demand: Tuesday, December 17, 2024 , at 9am EST (3pm CET / EU-Central) Register for this free webinar to explore how next-generation RNA technologies can provide effective solutions for vaccine innovation and gene therapy.
XTalks
JANUARY 9, 2023
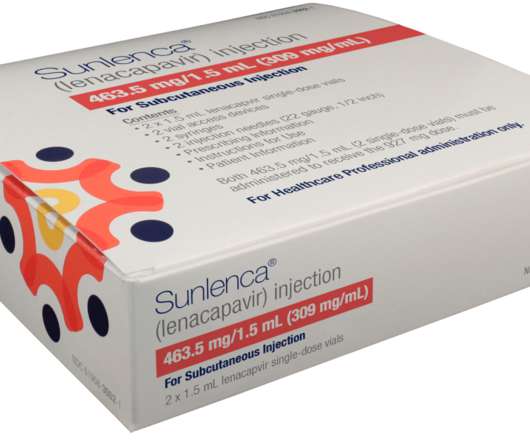
log10 copies/mL reduction in HIV-1 RNA from baseline at the end of the functional monotherapy period. Thirty-six participants were randomly allocated to receive either oral lenacapavir or placebo in a 2:1 ratio for 14 days, while continuing their failing regimen (functional monotherapy).
XTalks
JANUARY 3, 2025
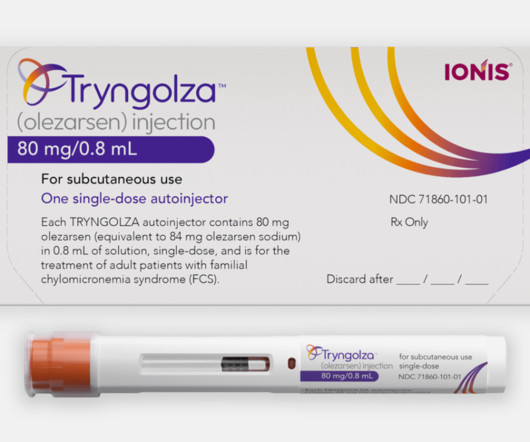
For the first time in the US, adults living with familial chylomicronemia syndrome (FCS) have an FDA-approved treatment option. In clinical trials, it showed up to an 86 percent reduction in triglyceride levels.

XTalks
DECEMBER 22, 2021
The RNA Revolution: From mRNA Vaccines to RNA Editing. The age of RNA is officially here, and it’s here to stay as more than a passing life science trend. RNA technology is not new nor has its potential been surprising. RNA in the Making. So why did this perceived RNA ‘revolution’ take so long?

BioTech 365
MAY 24, 2021
Scopus BioPharma Announces FDA Approval of IND Application for Lead Drug Candidate Scopus BioPharma Announces FDA Approval of IND Application for Lead Drug Candidate CpG-STAT3siRNA is a Distinctive RNA Therapy and Immunotherapy Developed at City of Hope A Phase 1 … Continue reading →

pharmaphorum
NOVEMBER 24, 2020
The FDA has approved Alnylam’s gene silencing drug Oxlumo, the first treatment for primary hyperoxaluria type 1 (PH1), an ultra-rare and life-threatening genetic disorder. The Swiss pharma’s bet looks to have paid off as inclisiran is likely to be approved in Europe in the coming weeks after backing from CHMP regulators last month.
XTalks
AUGUST 18, 2023
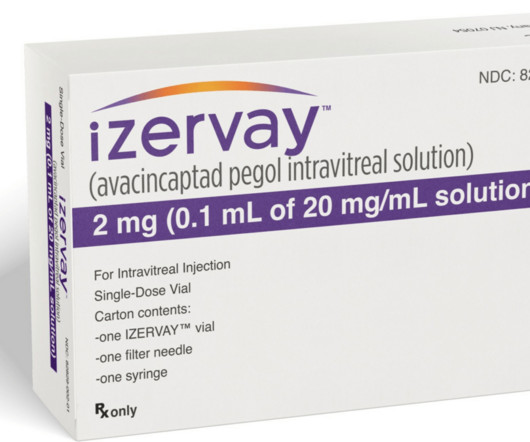
The FDA approval of Izervay is great news for the retina community and our patients suffering from geographic atrophy,” said Arshad M. Avacincaptad pegol combines an RNA aptamer with a roughly 43-kiloDalton (kDa) branched polyethylene glycol (PEG) molecule. How Does Izervay Work?

pharmaphorum
JANUARY 10, 2022
Previously, messenger RNA (mRNA) therapies were a niche part of the global R&D pipeline, now a wide section of the public is at least familiar with the name of this type of therapy. Pfizer already has a head start on competitors in the field, having achieved the first US FDA approval for an mRNA vaccine, alongside BioNTech.

The Pharma Data
JANUARY 21, 2021
Today’s FDA approval of Cabenuva represents a shift in the way HIV is treated, offering people living with HIV a completely new approach to care,” said Lynn Baxter, Head of North America, ViiV Healthcare. “Not At the same time, the FDA approved ViiV’s New Drug Application (NDA) for Vocabria (cabotegravir) 30 mg oral tablets.
XTalks
OCTOBER 7, 2020
While the new test is based on RT-PCR molecular detection, it utilizes a high-throughput technique involving a new heat-based extraction method and enhanced technology to extract RNA from samples for COVID-19 testing. LabCorp was given the green light for the test by the FDA last week, which is set to be rolled out soon.
XTalks
JANUARY 25, 2021
Patients in the studies were virologically suppressed (HIV-1 RNA less than 50 copies/milliliter) prior to initiation of treatment with Cabenuva. Cabenuva was actually expected to win FDA approval towards the end of 2019; however, manufacturing issues garnered rejection from the FDA at the time. Opening up Options.
The Pharma Data
JANUARY 22, 2021
Approval was based on data from the Phase 3 ATLAS and FLAIR studies, two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults. Before initiating Cabenuva treatment, study participants were virologically suppressed (HIV-1 RNA <50 copies/mL).

XTalks
NOVEMBER 30, 2023
New FDA-Approved Treatments For HIV HIV treatment involves the administration of combined antiretroviral therapy (ART) to effectively suppress the viral load, maintain or enhance immune function and reduce the risk of opportunistic infections and cancers commonly associated with HIV. aiming to end the HIV epidemic by 2030.
Pharmaceutical Technology
NOVEMBER 22, 2022
326 days after SARS-CoV-2 was first sequenced, the FDA approved Pfizer and BioNTech’s Comirnaty® under Emergency Use Authorization (EUA). Messenger RNA vaccines contain nucleic acids that code for a specific protein, or target antigen, related to a virus or disease. million in funding to support this work.

The Pharma Data
FEBRUARY 25, 2022
Several FDA-approved drugs – including for type 2 diabetes, hepatitis C and HIV – significantly reduce the ability of the Delta variant of SARS-CoV-2 to replicate in human cells, according to new research led by scientists at Penn State. ” The findings published today (Feb.

The Pharma Data
NOVEMBER 24, 2020
FDA Approves Oxlumo (lumasiran) for the Treatment of Primary Hyperoxaluria Type 1. Food and Drug Administration (FDA) approved Oxlumo (lumasiran) injection for subcutaneous use, the first-ever therapy available for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary oxalate levels in pediatric and adult patients.
XTalks
JUNE 5, 2023
RSV belongs to the family of negative-strand RNA viruses and was first identified in 1955. Today’s FDA approval of Abrysvo recognizes significant scientific progress, and importantly helps provide older adults potential protection against RSV and an opportunity to improve community health by helping prevent the disease,” said Edward E.
The Pharma Data
NOVEMBER 18, 2020
Food and Drug Administration has said that coronavirus vaccines should be at least 50 percent effective to be approved. Both vaccine makers used a synthetic version of coronavirus genetic material called messenger RNA to program a person’s cells to churn out copies of a fragment of the virus, the Times said.

The Pharma Data
JANUARY 21, 2021
In the ATLAS study, CABENUVA met the primary endpoint for noninferiority (the proportion of participants with plasma HIV-1 RNA ?50 50 copies per milliliter [c/mL] at Week 48), with a comparable number of patients receiving either CABENUVA or their daily current antiretroviral regimen (CAR) having an HIV-1 RNA level ?50
pharmaphorum
MARCH 4, 2021
billion for its FDA-approved dry eye drug Xiidra during the Japanese pharma’s merger with Shire in 2019, outlining the sales potential in this market niche. Sylentis, part of PharmaMar Group, has just announced FDA approval of a phase 3 trial for eye drops containing tivanisiran, in dry eye disease associated with Sjogren’s Syndrome.
XTalks
MAY 5, 2023
This week, the US Food and Drug Administration (FDA) approved the world’s first respiratory syncytial virus (RSV) vaccine. The shot, named Arexvy, is approved for adults aged 60 years and older and was developed by GlaxoSmithKline (GSK). RSV is a negative-strand RNA virus that was first identified in 1955.

XTalks
SEPTEMBER 4, 2024
Alnylam Pharmaceuticals announced promising results from its HELIOS-B Phase III clinical trial evaluating vutrisiran, an investigational RNA interference (RNAi) therapeutic for treating transthyretin amyloidosis with cardiomyopathy (ATTR-CM). As of March 2024, the FDA has approved six small interfering RNA (siRNA) therapies.

Pharmaceutical Technology
FEBRUARY 1, 2023
RSV researchers at major pharmaceutical companies are currently working to develop new RSV drugs to beat future waves of RSV infection and gain the first RSV vaccine FDA approval. Moderna brings up the rear after announcing Phase III results with its own messenger RNA (mRNA) RSV vaccine earlier this month. per 100,000.

XTalks
JUNE 14, 2023
More Drugs for Dry Eye Disease Are on the Way Several other drugs have recently gained FDA approval or are currently in advanced stages of clinical development for the treatment of dry eye disease. One such approved medication is Miebo , an ophthalmic solution developed by Bausch + Lomb and Novaliq.

pharmaphorum
DECEMBER 23, 2021
At its second attempt, Novartis has won FDA approval for its cholesterol lowering drug inclisiran, which can reduce levels with just two injections a year. Novartis resubmitted its marketing application in the US earlier this year, after swapping fill-and-finish production to one of its own facilities in Schaftenau, Austria.
XTalks
JULY 10, 2024
Unlike traditional testing methods that require samples to be sent to central laboratories, this test utilizes a blood sample from the fingertip to detect HCV RNA, delivering results in approximately one hour. “This is the first HCV RNA detection technology sensitive enough for active case finding at the point of care.

The Pharma Data
AUGUST 31, 2020
Food and Drug Administration (FDA) approval for the cobas® HIV-1/HIV-2 Qualitative Test for use on the fully automated cobas® 6800/8800 Systems in the U.S. Combines confirmatory HIV testing and HIV-1/HIV-2 differentiation into one single test. Basel, 1 September 2020 – Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced U.S.

XTalks
OCTOBER 26, 2023
The US Food and Drug Administration (FDA) approved new updated COVID-19 vaccines from both Pfizer-BioNTech and Moderna that target the Omicron XBB.1.5 In May, the FDA approved GSK’s Arexvy as the first RSV vaccine, which was shortly followed by the approval of Pfizer’s RSV vaccine Abrysvo.
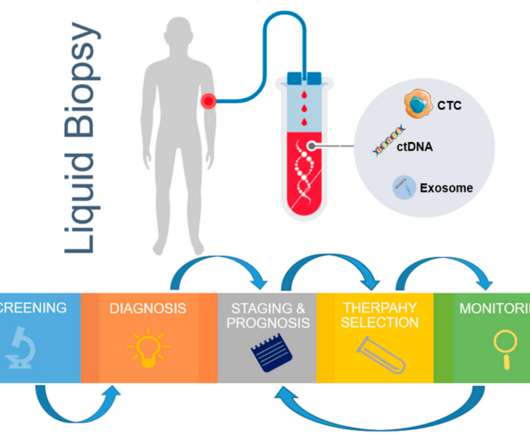
XTalks
NOVEMBER 7, 2022
Immunofluorescent staining of CTCs is a CTC isolation method that has been used since the first US Food and Drug Administration (FDA) approval in 2013 for the CellSearch ® CTC enumeration platform. Regulatory Approvals for CTC-Based Assays in Oncology. Table 1: Some examples of cfDNA-based assays that are approved by the US FDA.

Worldwide Clinical Trials
MAY 30, 2024
From drugs previously FDA-approved for gallstone diseases, Parkinson’s disease, and even cancer, researchers are working tirelessly to test and establish whether these pharmacotherapies with well-defined safety profiles may have any potential for efficacy in treating ALS.

XTalks
MAY 10, 2023
In this episode, Ayesha talked about the FDA approval of the world’s first vaccine for respiratory syncytial virus (RSV). The vaccine is approved for adults 60 years of age and older. Ayesha and the team also discussed the FDA approval of Qalsody (tofersen), a novel, first-of-its-kind treatment for ALS.

pharmaphorum
DECEMBER 23, 2020
There was some steady progress in neurology – in February FDA approved Lundbeck’s eptinezumab prophylactic treatment for migraine, the last from a gang of four drugs from a new class. It was the third approval from Alnylam’s pipeline of RNA interference therapeutics to make it to market. months, compared with 6.7

The Pharma Data
DECEMBER 8, 2020
Laying down a new track for RNA processing, Remix launched with $81 million in financing. Funds will be used to support development of the REMaster technology platform and advance the company’s pipeline of RNA processing targeted therapeutics as well. “As Pear Therapeutics.

The Pharma Data
FEBRUARY 12, 2022
Veklury was approved by the FDA on October 22, 2020 for grown-ups and pediatric cases 12 times of age and aged and importing at least 40 kg for the treatment of COVID-19 taking hospitalization. Veklury directly inhibits viral replication inside of the cell by targeting the SARS-CoV-2 viral RNA polymerase.

pharmaphorum
APRIL 5, 2022
.” Strong efficacy data is likely to be the key to carving out a role for the antisense drug in the PCSK9 inhibitor class, particularly as it is up against some heavyweight competition – notably Novartis’ small interfering RNA (siRNA) therapy Leqvio (inclisiran), which only needs to be administered twice a year.

pharmaphorum
JULY 19, 2021
Drugs harnessing the potential of modified RNA, antisense and siRNA oligonucleotides, antibody–drug conjugates and nanomedicines will become as important as today’s ‘small molecule’ drugs, reaching potential market values into the hundreds of billions – if the potential can be realised through innovation support and industry collaboration.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content