Novavax seeks FDA approval for updated Covid-19 vaccine
Pharmaceutical Technology
JUNE 17, 2024
Novavax has sought US FDA approval for an updated JN.1 1 version of its Covid-19 vaccine, NVX-CoV2705, for individuals aged 12 years and above.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmaceutical Technology
JUNE 17, 2024
Novavax has sought US FDA approval for an updated JN.1 1 version of its Covid-19 vaccine, NVX-CoV2705, for individuals aged 12 years and above.

Pharmaceutical Technology
MAY 10, 2024
The FDA approved WestGene’s mRNA therapeutic cancer vaccine as mRNA cancer vaccine development rises in popularity.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MARCH 24, 2025
The FDA has granted approval for the IND application of Everest Medicines tumour-associated antigen (TAA) vaccine, EVM14.

Bio Pharma Dive
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.
Bio Pharma Dive
MAY 31, 2023
The shot’s clearance comes several weeks after the regulator made GSK’s Arexvy the first vaccine for RSV in the U.S.
Pharmaceutical Technology
JUNE 3, 2024
Moderna has secured approval from the US Food and Drug Administration (FDA) for mRESVIA (respiratory syncytial virus vaccine).

Bio Pharma Dive
MAY 7, 2021
The milestone filing could pave the way for the shot's use beyond the pandemic and give employers the legal heft to require vaccination, a key step toward herd immunity in the U.S.

Pharmaceutical Technology
SEPTEMBER 23, 2024
The US FDA has granted approval for AstraZeneca's FluMist, a needle-free nasal spray influenza vaccine for self-administration.
Pharmaceutical Technology
SEPTEMBER 12, 2023
The US FDA has approved Pfizer and BioNTech’s sBLA for Omicron XBB.1.5-adapted adapted monovalent Covid-19 vaccine, COMIRNATY 2023-2024 Formulation.

Pharmaceutical Technology
AUGUST 4, 2023
The US FDA has granted approval for an expanded indication of Merck’s Ebola vaccine, Ervebo, for usage in the paediatric population.

Bio Pharma Dive
MAY 3, 2023
The decision represents the first fruits of a scientific breakthrough a decade ago that gave drugmakers, among them GSK, Pfizer and Moderna, a blueprint for an effective shot against the virus.

Bio Pharma Dive
MARCH 29, 2021
A long-awaited reckoning for Biogen’s Alzheimer’s drug and the review of AstraZeneca’s coronavirus vaccine are among the top FDA decisions expected before the end of June.

Bio Pharma Dive
SEPTEMBER 29, 2020
The world's attention will be on the FDA as it considers initial data from coronavirus vaccine developers. But several other important drugs, including a CAR-T therapy and an Ebola antibody, will also be on the agency's agenda.

Bio Pharma Dive
SEPTEMBER 11, 2023
Pfizer and Moderna, which have seen slowing demand for their coronavirus vaccines, expect to make the reformulated shots available in the U.S. in the coming days.

Bio Pharma Dive
OCTOBER 1, 2021
Regulators face key decisions on COVID-19 shots for children and boosters for Moderna's and J&J's vaccines. Other closely watched drugs for multiple myeloma and depression are under review, too.

XTalks
NOVEMBER 15, 2024
Pfizer released a new respiratory syncytial virus (RSV) vaccine TV commercial, titled “Your Moments Are Worth Protecting: Celebration” as part of its ongoing campaign to raise awareness about the importance of vaccination against RSV. Moderna entered the RSV vaccine arena this year with the very first mRNA-based RSV vaccine.
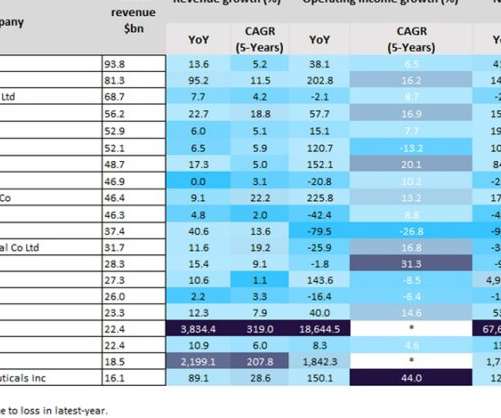
Pharmaceutical Technology
SEPTEMBER 15, 2022
Last year was a positive year for biopharmaceutical companies, particularly those with Covid-19 vaccines. As a result of huge global sales of mRNA Covid-19 vaccines, the split in profits between Pfizer and BioNTech’s Comirnaty contributed towards revenues of $81.3bn and $22.4bn last year, respectively. YoY revenue growth.

Bio Pharma Dive
JULY 11, 2023
While the shot is approved in the EU, Takeda wasn’t able to address data collection issues raised by the US regulator in its current review cycle.

Bio Pharma Dive
AUGUST 21, 2023
The expanded approval follows the FDA’s May clearance of the shot, called Abrysvo, in older adults.

Pharmaceutical Technology
OCTOBER 10, 2022
The US Food and Drug Administration (FDA) has approved GlaxoSmithKline ’s (GSK) Boostrix (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed [Tdap]) for pregnant women during their third trimester to prevent pertussis (whooping cough) in newborn infants.

Pharmaceutical Technology
FEBRUARY 17, 2025
GSK has secured approval from the FD for its new vaccine, Penmenvy, to protect against five meningococcal serogroups.
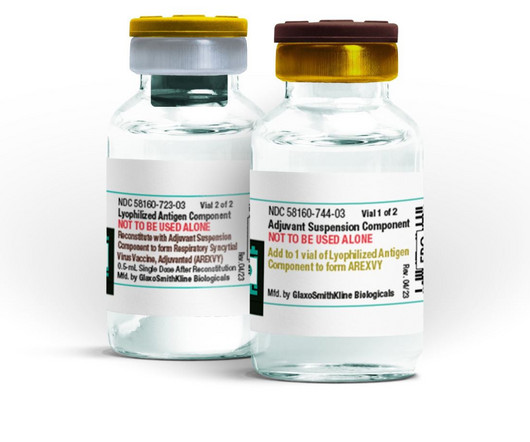
Pharmaceutical Technology
JUNE 10, 2024
The US FDA approved GSK's Arexvy, an RSV vaccine for adults aged 50 to 59 years at higher lower respiratory tract disease (LRTD) risk.
Pharmaceutical Technology
AUGUST 23, 2024
The US FDA has approved and granted EUA for Moderna and Pfizer-BioNTech’s updated mRNA Covid-19 vaccines, Comirnaty and Spikevax.

Pharma Times
SEPTEMBER 18, 2023
The updated vaccines more closely target current circulating variants - News - PharmaTimes
Pharmaceutical Technology
APRIL 1, 2025
The formulation allows for greater stockpiling, with Bavarian Nordic already working with the US government to add to vaccine reserves.

Bio Pharma Dive
JUNE 30, 2022
But the drug may not meaningfully help people who are vaccinated, and concerns about its potency against emerging variants are growing. Converting Paxlovid’s emergency authorization to a standard clearance could further broaden its use.

Pharmaceutical Technology
NOVEMBER 10, 2023
Following the three-month FDA review delay, Valneva’s live-attenuated chikungunya vaccine, Ixchiq received accelerated approval.

Bio Pharma Dive
FEBRUARY 19, 2021
Testing showed the vaccine could be safely stored at normal freezer temperatures, rather than the roughly minus 70 degrees Celsius now specified. The change, if cleared by the agency, will help distribution.

Pharmaceutical Technology
AUGUST 30, 2024
Emergent BioSolutions has received FDA approval for ACAM2000 to expand its use for the prevention of Mpox.

Bio Pharma Dive
SEPTEMBER 9, 2020
Results from two Phase 3 studies put Merck in position to ask for FDA approval of its shot later this year. Pfizer may not be far behind with a rival product.

XTalks
JANUARY 22, 2025
Valneva SE, who developed the worlds first FDA-approved chikungunya vaccine , has now unveiled promising Phase III data for its single-shot chikungunya vaccine, IXCHIQ, in adolescents aged 12 to 17. The VLA1553-321 trial, conducted in Brazil, was the first to assess the vaccine in an endemic region.
XTalks
JUNE 21, 2024
The US Food and Drug Administration (FDA) has green-lighted the first pneumonia vaccine specifically designed for adults 50 years of age and older. These numbers do not reflect the efficacies of the vaccines, which have not been compared head-to-head in any trial yet.
XTalks
DECEMBER 7, 2021
VBI Vaccines got its first vaccine approval from the US Food and Drug Administration (FDA) for its hepatitis B shot PreHevbrio. PreHevbrio is approved for the prevention of hepatitis B infection caused by the hepatitis B virus (HBV) in adults 18 years of age and older.
pharmaphorum
MAY 31, 2024
Moderna has secured FDA approval for its RSV vaccine, setting up a three-way market battle with GSK and Pfizer.

Pharmaceutical Technology
OCTOBER 28, 2022
On 24 October, American vaccine developer Vaxcyte shared positive topline data from a Phase I/II study of its multivalent conjugate pneumococcal vaccine VAX-24 , bringing the 24-valent pneumococcal jab one step closer to market. This is in addition to its long-available pneumococcal polysaccharide vaccine PPSV23.
XTalks
JULY 25, 2023
Emergent BioSolutions, a multinational specialty biopharmaceutical company headquartered in Gaithersburg, Maryland, has achieved a significant milestone with the approval of its Cyfendus (Anthrax Vaccine Adsorbed, Adjuvanted) vaccine by the US Food and Drug Administration (FDA). How Does Cyfendus Work?

Pharmaceutical Technology
FEBRUARY 1, 2023
RSV researchers at major pharmaceutical companies are currently working to develop new RSV drugs to beat future waves of RSV infection and gain the first RSV vaccine FDA approval. Pharmaceutical companies are pushing to develop drugs and vaccines for RSV with these populations in mind. per 100,000.

Pharmaceutical Technology
APRIL 28, 2023
The US Food and Drug Administration (FDA) has approved Pfizer’s 20-valent pneumococcal conjugate vaccine, PREVNAR 20 , to prevent invasive pneumococcal disease (IPD) in infants and children aged six weeks to 17 years. PREVNAR 20 has been developed on the basis of Pfizer’s approved PREVNAR 13 vaccine.
XTalks
JULY 28, 2021
Merck has scored US Food and Drug Administration (FDA) approval for its next-generation pneumonia vaccine Vaxneuvance that covers 15 different strains of the pneumococcal bacteria that causes the infection. This is seven more strains than its current winning vaccine Prevnar 13, which registered $5.95
Fierce Pharma
JUNE 17, 2024
The FDA has approved the world’s first pneumococcal disease vaccine designed for adults, signing off on Merck’s Capvaxive (formerly V116) and positioning it to become the primary shot used by senio | The FDA has approved the world’s first pneumococcal disease vaccine designed for adults, signing off on Merck’s Capvaxive (formerly V116) and positioning (..)

Bio Pharma Dive
JANUARY 18, 2023
Moderna plans to follow those companies in asking for FDA approval. The biotech’s results appear competitive to past data for RSV shots being developed by GSK and Pfizer.

BioSpace
MAY 6, 2021
FDA for COVID-19 vaccines, Pfizer/BioNTech and Moderna are planning to seek full approval for the preventative medications. Six months after receiving Emergency Use Authorization from the U.S.

pharmaphorum
AUGUST 30, 2024
Emergent BioSolutions has been given FDA approval to extend the indications for its smallpox vaccine ACAM2000 to include the prevention of mpox, currently deemed a public health emergency by the WHO.

BioSpace
JUNE 21, 2022
On Wednesday, the FDA approved Merck's pneumococcal 15-valent conjugate vaccine for children 6 weeks through 17 years of age.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content