Comprehensive Guide to Medical Device Safety, Systems, and Regulatory Compliance: FAQ
Cloudbyz
AUGUST 19, 2024
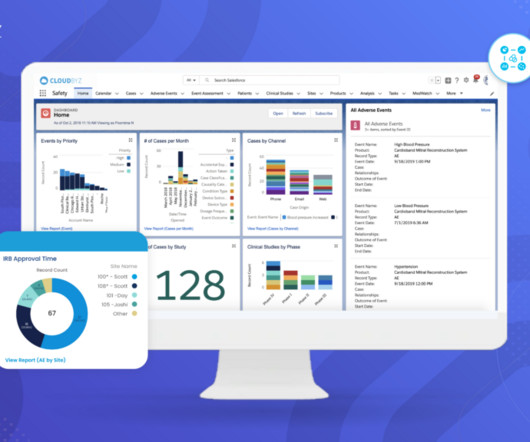
Introduction to Medical Device Safety, Systems, and Regulations The medical device industry is a rapidly evolving field that plays a critical role in modern healthcare. This is where the importance of stringent safety processes, robust systems, and comprehensive regulations comes into play. What is the Medical Device Safety process?










Let's personalize your content