Anocca signs licensing deal for EmendoBio’s gene-editing tech
Pharmaceutical Technology
MARCH 15, 2024
Anocca has entered a licensing agreement with EmendoBio for the use of the latter’s OMNI-A4 nuclease, a gene editing technology.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MARCH 15, 2024
Anocca has entered a licensing agreement with EmendoBio for the use of the latter’s OMNI-A4 nuclease, a gene editing technology.
Bio Pharma Dive
MARCH 2, 2022
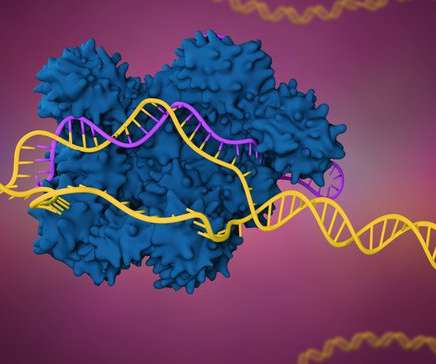
A federal patent board ruled Broad Institute scientists were first to a key gene editing advance, weakening the patent position of Intellia and CRISPR Therapeutics, which hold licenses through the University of California.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Bio Pharma Dive
MARCH 27, 2023
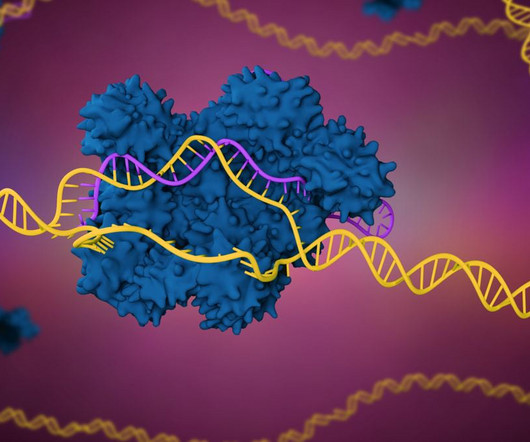
Vertex will license CRISPR technology to develop insulin-producing islet cells that are more resistant to immune rejection, adding to other efforts directed at Type 1 diabetes.

Bio Pharma Dive
OCTOBER 22, 2024
The gene editing company will focus on “in vivo” medicines, while seeking to license out or find a development partner for its clinical-stage treatment reni-cel.
Worldwide Clinical Trials
NOVEMBER 27, 2023
Casgevy, the commercial product formerly known as exa-cel, is administered by taking stem cells out of a patient’s bone marrow and editing a gene in the cells in a laboratory, with the modified cells then infused back into the patient after conditioning treatment to prepare the bone marrow. In June 2023, the U.S.

Bio Pharma Dive
JANUARY 19, 2023
Shoreline will acquire a preclinical NK cell therapy Editas has been developing, as well as a license to use the CRISPR biotech’s gene editing technology.

Pharmaceutical Technology
DECEMBER 14, 2023
The payment, which encompasses newly approved Casgevy, is the latest deal in a long line of CRISPR licensing twists and turns.

Bio Pharma Dive
OCTOBER 4, 2024
The gene editing company is selling to DRI Healthcare Trust future license fees that are owed to it under an agreement with Vertex last year.

Pharmaceutical Technology
MARCH 28, 2023
Vertex Pharmaceuticals has signed a new non-exclusive licensing agreement with CRISPR Therapeutics to expedite the development of its hypoimmune cell therapies to treat type 1 diabetes (T1D). The gene-editing technology allows for precise, directed changes to genomic DNA. The system comprises the Cas9 enzyme and a guide RNA.

pharmaphorum
JANUARY 10, 2024
Precision BioSciences licenses non-cancer uses of its allogeneic CAR-T therapy azer-cel to TG Therapeutics as it pivots to in vivo applications of its gene-editing technology

pharmaphorum
OCTOBER 8, 2020
Scribe Therapeutics, a start-up focusing on gene-editing using CRISPR/Cas9, has burst onto the biotech scene with a $415 million deal with Biogen. The big biotech meanwhile has an antisense drug in trials for a subtype of inherited ALS, licensed from Ionis Pharma, in phase 3 clinical trials. Jennifer Doudna.

BioTech 365
MARCH 2, 2021
ERS Genomics Licenses CRISPR Gene Editing Technology to Otsuka Pharmaceutical ERS Genomics Licenses CRISPR Gene Editing Technology to Otsuka Pharmaceutical License to Nobel prize winning CRISPR technology supports internal research and development DUBLIN, Ireland–(BUSINESS WIRE)–ERS Genomics Limited, which was formed … Continue reading (..)

BioTech 365
DECEMBER 23, 2020
Arbor Biotechnologies and Lonza Enter into Gene Editing Technology Licensing Deal Arbor Biotechnologies and Lonza Enter into Gene Editing Technology Licensing Deal The new agreement will provide Lonza with access to Arbor’s gene editing technology Quote from David Cheng, CEO … Continue reading →
Pharmaceutical Technology
APRIL 28, 2023
EDIT-301 is made of patient-derived CD34+ haematopoietic stem and progenitor cells, which are edited using CRISPR at the gamma globin gene (HBG1 and HBG2) promoter sites using a proprietary engineered AsCas12a nuclease, per the company’s website. Shoreline acquired EDIT-202 as part of this agreement.

BioTech 365
OCTOBER 19, 2021
Sana Biotechnology Obtains a Non-Exclusive License to CRISPR Cas12b Gene Editing Technology from Beam Therapeutics to Enable Engineered Cell Programs Sana Biotechnology Obtains a Non-Exclusive License to CRISPR Cas12b Gene Editing Technology from Beam Therapeutics to Enable Engineered Cell Programs … Continue reading →

Drug Discovery Today
DECEMBER 2, 2020
Sanyou signs two commercial-use licenses for Horizon’s gene-edited CHO-K1 GS knockout cell line, for use in biotherapeutic pipeline development and contract research services

BioSpace
DECEMBER 12, 2023
The deal follows the FDA approval of Vertex’s gene-editing sickle cell treatment and Editas’ earlier legal battle over rights to the technology.

The Pharma Data
NOVEMBER 30, 2020
Vivlion holds an exclusive license to Goethe University of Frankfurt’s proprietary 3Cs technology for the production of next generation 3Cs CRISPR/Cas gRNA libraries. The license from ERS Genomics now enables Vivlion to offer both R&D reagents and screening services to its customers worldwide. “The

BioTech 365
FEBRUARY 16, 2021
ERS Genomics and ZeClinics Sign CRISPR/Cas9 License Agreement ERS Genomics and ZeClinics Sign CRISPR/Cas9 License Agreement ZeClinics is applying CRISPR technology to create gene edited zebrafish disease models DUBLIN & BARCELONA, Spain–(BUSINESS WIRE)–ERS Genomics Limited, which was formed to provide … Continue reading → (..)
pharmaphorum
FEBRUARY 19, 2021
Snipping out this viral code with powerful CRISPR gene editing technology, which last year won Drs Emmanuelle Charpentier and Jennifer Doudna the Nobel Prize for chemistry, would in theory prevent the need for drugs to suppress the virus and the development of AIDS.
Pharmaceutical Technology
MARCH 9, 2023
In 2022, Immatics and Editas Medicine entered a research collaboration and licensing agreement to combine gamma-delta T-cell adoptive cell therapies and gene editing to develop medicines for the treatment of cancer. Bristol-Myers Squibb and Cellectis are the other key patent filers in in-vitro T-cell activation.
pharmaphorum
SEPTEMBER 29, 2020
For example, under a traditional pharma/biotech collaboration and licensing model, a biotech partner may have primary responsibility for significant elements of research and early product development, and the pharma partner may lead the majority of later stage development, as well as post-approval commercial manufacture and supply.
pharmaphorum
MAY 7, 2021
Gene editing firm CRISPR Therapeutics has announced a collaboration with US biotech Nkarta to develop natural killer (NK) cell therapies for cancer. It’s at the forefront of gene editing although the technology has spawned rivals including Intellia Therapeutics, Caribou Biosciences, Sangamo Therapeutics and Mammoth Biosciences.

Pharmaceutical Technology
JANUARY 19, 2023
Despite the challenges faced by gene-modified cell therapies such as CAR-T cells, significant promise remains for allogeneic cell therapies that have not been gene edited. NiCord’s biologics license application is currently being investigated by the FDA with a review date set by May 2023.

Pharmaceutical Technology
APRIL 25, 2023
After missing the previous Q1 deadline, bluebird bio has submitted a Biologics License Application (BLA) to the US Food and Drug Administration (FDA) for its sickle cell disease gene therapy lovo-cel, based on an April 24 company announcement.

BioTech 365
OCTOBER 13, 2020
Food analysis and biotechnology company licenses gene editing technology DUBLIN & KANAGAWA, Japan–(BUSINESS WIRE)–ERS Genomics Limited (“ERS”), which was formed to provide broad access to the foundational CRISPR/Cas9 intellectual property (IP) co-owned by Dr. Emmanuelle Charpentier, today announced it has … Continue (..)

pharmaphorum
JANUARY 10, 2022
Bayer has bolstered its cell and gene therapy platform by securing access to a CRISPR-based gene-editing platform developed by US biotech Mammoth Biosciences.
XTalks
JANUARY 4, 2024
There is a significant focus on developing gene therapies as longer-term solutions for the disease. Pfizer licensed Beqvez from Spark Therapeutics nine years ago. Beqvez is set to rival CSL Behring’s Hemgenix (etranacogene dezaparvovecfor), which was the first gene therapy approved for hemophilia B. Priced at $3.5

The Pharma Data
AUGUST 4, 2020
UK-based Oxford Biomedica has signed a new development, manufacture and license agreement with Beam Therapeutics for next-generation CAR-T therapeutics. The agreement grants Beam a non-exclusive license to Oxford Biomedica’s LentiVector platform, for tis application in next-generation CAR-T programmes in oncology. .

The Pharma Data
JUNE 7, 2023
Bayer strengthens gene therapy portfolio with lipid nanoparticle technology from Acuitas Therapeutics Bayer AG is joining forces with Acuitas Therapeutics, Inc., “Accessing state-of-the-art LNP technology through this collaboration will add momentum to our gene editing efforts for the benefit of patients.”

XTalks
MAY 22, 2023
Related: 5 Food Companies Working With Precision Fermentation Technology How Pairwise Leverages CRISPR Technology CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is a gene-editing technique that can be used to alter the DNA of cells to enhance certain characteristics or reduce less desirable ones.

pharmaphorum
SEPTEMBER 28, 2022
” ArsenalBio’s platform makes use of automation, large-scale genome engineering, using technologies like CRISPR-based gene-editing, and machine learning and artificial intelligence algorithms to design, build, and test cell therapies.

The Pharma Data
JANUARY 12, 2021
Marianne De Backer, Head of Business Development & Licensing in Bayer’s Pharmaceuticals Division, pictured above. In an interview with BioSpace, De Backer outlined Bayer’s thought process for opening its purse strings and diving into the deep end of the cell and gene therapy space. Photo courtesy of Bayer.

pharmaphorum
DECEMBER 21, 2022
As part of the acquisition, Kite will assume responsibility for continuing the R&D collaboration between Tmunity and Penn, which includes options and licenses to cell engineering and manufacturing technologies invented and developed in the university’s laboratories. Image by Anja from Pixabay .

The Pharma Data
DECEMBER 2, 2020
ERS Genomics – Ireland’s ERS Genomics Limited, and Germany’s Vivlion GmbH, announced a non-exclusive license agreement granting Vivlion access to ERS Genomics’ CRISPR/Cas9 patent portfolio, to enhance Vivlion’s gene editing reagents and screening services. Horizon Discovery Group – U.K.-based in Mainland China.

BioSpace
NOVEMBER 17, 2022
Editas Medicine is pausing its ocular gene therapy program after demonstrating a favorable safety profile and seeking a potential partner to develop EDIT-101, the company announced Thursday.

pharmaphorum
JUNE 17, 2022
Other programmes for the disease that have fallen by the wayside in recent years include C9ORF72-targeitng antisense drug BIIB078 , which failed a trial reported in March, and type 2B activin receptor antagonist BIIB110, licensed from AliveGen. The post Biogen waves bye to $217m ALS partnership with Karyopharm appeared first on.

Delveinsight
MARCH 2, 2021
In return, HitGen will receive an upfront payment, success fee, license/milestone fee from UPPTHERA, details of which remain undisclosed. HitGen plans to apply its platform technology based on the design, synthesis, and screening of DNA-encoded libraries (DEL) for the discovery of compounds to bind to specific targets.
The Pharma Data
MARCH 11, 2021
Second, the biorevolution is driving innovation in all of Bayer’s divisions – with major progress in cell biology, gene editing and data science. Last year alone, the company concluded more than 25 collaboration and licensing agreements and acquisitions in its Pharmaceuticals Division.

The Pharma Data
NOVEMBER 3, 2020
It may also allow for priority or rolling review of a company’s Biologics License Application (BLA). LogicBio’s proprietary genome editing technology platform, GeneRide, enables the site-specific integration of a therapeutic transgene without nucleases or exogenous promoters by harnessing the native process of homologous recombination.
The Pharma Data
JANUARY 24, 2021
CYAD-101 is the company’s allogeneic NKG2D-receptor and T cell receptor inhibitory molecule-based, non-gene edited CAR-T candidate. The drug was licensed from Immutep Limited. Celyad Oncology updated its Phase I alloSHRINK trial of CYAD-101 for refractory metastatic colorectal cancer.

XTalks
DECEMBER 29, 2022
In 2022 alone, the US regulator approved four new gene therapies, showing the high interest in getting these therapies to market. In 2023, a number of gene therapies are expected to get the FDA green light. The therapy, exagamglogene autotemcel (exa-cel), was granted a rolling review by the FDA.

XTalks
AUGUST 2, 2023
Late in 2022, AbbVie partnered with HotSpot Therapeutics and announced an exclusive worldwide collaboration and option to license agreement for their interferon regulatory factor 5 (IRF5) inhibitor program for autoimmune disease treatment. In 2022, the FDA proceeded to grant official licensing for Moderna’s Spikevax COVID-19 vaccine.

Pharmaceutical Technology
MAY 2, 2023
Immatics will receive $15m from the global license agreement and is in line for a further $490m in milestone payments for this candidate. Immatics also has a research collaboration with gene editing company Editas Medicine to advance its T cell therapies. Immatics’ CEO and co-founder.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content