Digital dilemma in UK’s life sciences job market
Pharmaceutical Technology
SEPTEMBER 8, 2023
In this issue: Digital jobs in UK life sciences, generative AI changes drug discovery, and how robotics is impacting gene therapy manufacturing.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
SEPTEMBER 8, 2023
In this issue: Digital jobs in UK life sciences, generative AI changes drug discovery, and how robotics is impacting gene therapy manufacturing.

XTalks
NOVEMBER 14, 2024
PTC Therapeutics has gained US Food and Drug Administration (FDA) approval for its new gene therapy, Kebilidi (eladocagene exuparvovec), for treating aromatic L-amino acid decarboxylase (AADC) deficiency.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
MAY 4, 2023
Forge Biologics and global life sciences company Labcorp have announced a strategic adeno-associated virus (AAV) gene therapy development and manufacturing collaboration. This will improve accessibility to services for AAV-mediated gene therapy programmes.

XTalks
MARCH 19, 2025
A 16-year-old patient died after treatment with Elevidys (delandistrogene moxeparvovec), Sarepta Therapeutics gene therapy for Duchenne muscular dystrophy (DMD). The patient suffered acute liver failure several months after receiving the therapy in December. Sarepta is reviewing all available data.

Bio Pharma Dive
MAY 6, 2021
Completion of the buyout eases concerns the arrangement might be held up by the FTC, which has signaled it will step up scrutiny of life sciences deals.

Pharmaceutical Technology
APRIL 3, 2023
The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.

Pharmaceutical Technology
MARCH 10, 2023
On 10 March, the National Health Service Blood and Transplant (NHSBT) opened a new Clinical Biotechnology Centre (CBC) with the aim of improving the UK’s ability to develop and manufacture cell and gene therapies. The opening of this new facility plays into the UK government’s Life Sciences Industrial Strategy.

Pharmaceutical Technology
MAY 24, 2023
Forge Biologics has joined the public-private collaboration, the Bespoke Gene Therapy Consortium (BGTC), to expedite the development and manufacture of new AAV [adeno-associated virus] gene therapies to treat patients with rare diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Pharmaceutical Technology
MAY 16, 2023
The Foundation for the National Institutes of Health (FNIH) has announced its plans to prioritise eight rare diseases to provide industry standards for manufacturing, preclinical testing and product analytical testing for gene therapy development. This will include pairing up indications with manufacturers amongst the BGTC’s partners.

Pharmaceutical Technology
DECEMBER 14, 2022
GSK has entered a strategic partnership with Wave Life Sciences to progress the discovery and development of oligonucleotide therapies for new genetic targets. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. By Cytiva Thematic.

XTalks
JUNE 14, 2023
Courtney Silverthorn who is an Associate Vice President at the Foundation for the National Institutes of Health (FNIH) and the Director of the Accelerating Medicines Partnership (AMP) program including the Bespoke Gene Therapy Consortium. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.
Pharmaceutical Technology
JUNE 16, 2023
The Abu Dhabi Department of Health (DoH) in the UAE has made a declaration of collaboration with Mass General Brigham’s (MGB) International Center for Genetic Disease (iCGD) to advance life sciences.
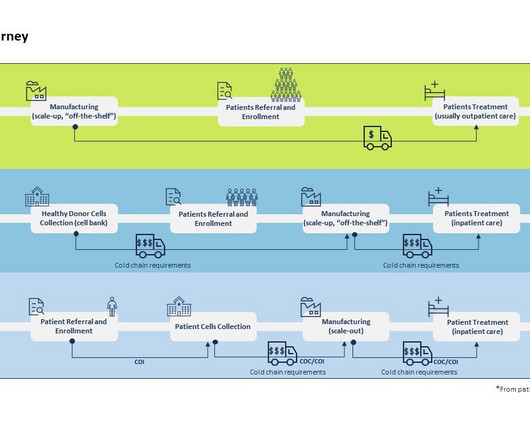
Pharmaceutical Technology
MAY 24, 2023
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.

Pharmaceutical Technology
DECEMBER 14, 2022
Merck and Synplogen have signed a non-binding Memorandum of Understanding (MoU) to expedite the development and manufacturing of viral vector-based gene therapy applications. The firms intend to merge their expertise to provide simplified viral vector gene therapy development, production and testing in Japan.

XTalks
OCTOBER 22, 2024
Canadian contract development and manufacturing organization (CDMO) OmniaBio has debuted its new state-of-the-art facility focused on cell and gene therapy (CGT) manufacturing. Focused on advancing regenerative medicine, CCRM supports the development of cell and gene therapies, along with the associated enabling technologies.

BioPharma Reporter
FEBRUARY 9, 2023
The Cell and Gene Therapy Catapult (CGT Catapult) has confirmed its involvement in the development of a new Â900m ($1,097m) life science campus in Stevenage, UK: which is set to become one of the largest in Europe.

XTalks
SEPTEMBER 12, 2023
Life science podcasts have emerged as an invaluable tool for building connections with audiences in the digital era. Furthermore, we’ll explore the unique advertising opportunities that this platform offers, positioning brands at the forefront of the life science industry. The result?

XTalks
MAY 17, 2023
The Foundation for the National Institutes of Health (FNIH) announced this week that the Accelerating Medicines Partnership Bespoke Gene Therapy Consortium (AMP BGTC) has selected eight rare diseases for its clinical trial portfolio. As such, rare disease patients and their families often face little hope for effective treatments.

XTalks
OCTOBER 15, 2024
Seven cases of blood cancer have been identified in new trial data for bluebird bio’s gene therapy Skysona (elivaldogene autotemcel). The patients had received the autologous hematopoietic stem cell-based gene therapy as a treatment for early-stage cerebral adrenoleukodystrophy (CALD), a rare and fatal neurodegenerative disorder.
XTalks
JANUARY 4, 2024
Pfizer has kickstarted the new year with its first-ever gene therapy approval, awarded by Health Canada to the company’s Beqvez (fidanacogene elaparvovec) for the treatment of hemophilia B. There is a significant focus on developing gene therapies as longer-term solutions for the disease.

pharmaphorum
OCTOBER 22, 2020
Sarepta chief commercial officer Bo Cumbo has left to head up gene therapy venture – AavantiBio – with $107 million in backing from his former employer and three high-profile life sciences investors. Unlike a one-shot gene therapy, omaveloxolone would require continuous dosing to maintain its effects.

XTalks
MARCH 25, 2021
Xtalks is proud to announce the launch of the Xtalks Life Science podcast. Subscribe to the Xtalks Life Science Podcast to never miss a new episode. Fresh Conversations About Life Science Topics. She focuses on news relating to the food industry and writes blogs on recruitment and HR in the life sciences.

XTalks
MARCH 8, 2022
On International Women’s Day, Xtalks is celebrating women’s leadership in the life sciences by highlighting some of the female leaders at the forefront of scientific discovery, as well as the continuing challenges of attaining more equitable representation. Challenges in Women’s Leadership.
XTalks
SEPTEMBER 9, 2021
The US Food and Drug Administration (FDA) has placed a clinical hold on BioMarin Pharmaceutical’s investigational gene therapy BMN 307 for the rare inherited disease phenylketonuria (PKU) over safety concerns found during preclinical testing. The mice developed the tumors one year after being given BMN 307.

pharmaphorum
AUGUST 31, 2022
After a recent approval, there are now three gene therapies available on the US market. In recent years, gene therapy has transitioned from a promising idea to a reality for patients, with many of the severe safety issues that emerged in early iterations of the technology being overcome. from 2021 to 2029.

XTalks
DECEMBER 20, 2023
As we step into 2024, the life sciences continue to evolve at an unprecedented pace, driven by technological innovation, a deeper understanding of human biology and the application of new technologies in areas like drug development and health wearables.

Cloudbyz
SEPTEMBER 10, 2023
The challenge of managing and securing sensitive data, including PHI and PII, is significant for life sciences organizations. Additionally, the consequences of data breaches can be severe for life sciences organizations. The challenge is further compounded by the need for fast and accurate analysis of data.

XTalks
MARCH 21, 2024
The US Food and Drug Administration (FDA) has awarded approval to Orchard Therapeutics for its gene therapy Lenmeldy (atidarsagene autotemcel) for the treatment of children with pre-symptomatic late infantile, pre-symptomatic early juvenile or early symptomatic early juvenile metachromatic leukodystrophy (MLD).

XTalks
DECEMBER 8, 2020
has announced that it has acquired the rights to an investigational gene therapy, HMR59, from Hemera Biosciences, LLC designed to help treat a severe form of age-related macular degeneration (AMD). The gene therapy was developed to treat both dry and wet macular degeneration. Janssen Pharmaceuticals Inc.

XTalks
APRIL 24, 2023
Awareness of rare diseases is growing, and with a better understanding of the pathophysiology of many rare diseases, innovative treatment options are emerging, like gene therapies that can treat the root cause of rare genetic diseases and potentially provide long-term symptom relief, or even a definitive cure.

XTalks
APRIL 14, 2021
In this episode of the Xtalks Life Science Podcast, Sarah revisits two Parkinson’s biotech companies — Prevail Therapeutics and Voyager Therapeutics — to see what progress they’ve made in their gene therapy clinical development programs since April 2019. Parkinson’s and Melanoma Share an Amyloid Link, Says New Research.

XTalks
JUNE 8, 2023
Over the past few years, there has been a significant expansion in the cell and gene therapy landscape, with an increasing number of therapies entering clinical trials and receiving regulatory approvals. “I think if we all go into these relationships with this mindset, we’ll achieve great things together.”

XTalks
AUGUST 19, 2022
Bluebird bio’s gene therapy Zynteglo (betibeglogene autotemcel, beti-cel) has been awarded a much anticipated approval from the US Food and Drug Administration (FDA) for the treatment of adult and pediatric patients with beta thalassemia who need regular blood transfusions. Bluebird has a total of three gene therapies in its pipeline.
XTalks
JUNE 30, 2023
It’s been a big week for cell and gene therapy approvals in the US, including a much-awaited approval for one to treat hemophilia A, the most common form of hemophilia. BioMarin said the majority of patients who received Roctavian received corticosteroids to suppress the immune system for the gene therapy to be effective and safe.
XTalks
DECEMBER 13, 2023
The US Food and Drug Administration (FDA) has approved the first gene therapies for the treatment of sickle cell disease, approving two on the same day. Both gene therapies are approved for individuals 12 years of age and older with sickle cell disease. How do Casgevy and Lyfgenia Work?

XTalks
JUNE 8, 2021
Novartis’ Zolgensma (onasemnogene abeparvovec) gene therapy has been making significant strides as of late, including dosing of the first Spinal Muscular Atrophy (SMA) patient with the treatment in the UK last week. Prior to Zolgensma, treatments could only help manage symptoms and attempt to improve quality of life.

XTalks
JANUARY 4, 2023
In this episode, Ayesha talked about some of the trends to look out for in the life sciences in 2023. Read the full article here: 4 Life Sciences Trends for 2023. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.

XTalks
AUGUST 30, 2022
The hemophilia A gene therapy Roctavian delivers a functional gene to enable the body to make an essential blood clotting protein on its own. Last week, the first gene therapy for severe hemophilia A , Roctavian (valoctocogene roxaparvovec), developed by BioMarin Pharmaceutical Inc.,
XTalks
MAY 26, 2023
Krystal Biotech’s Vyjuvek has been awarded US Food and Drug Administration (FDA) approval to make it the first topical gene therapy for the treatment of wounds in patients with the rare, often debilitating skin disease dystrophic epidermolysis bullosa (DEB). As a topical treatment, it is also the first readily redosable gene therapy.

XTalks
JUNE 28, 2023
Gene therapies for Duchenne muscular dystrophy (DMD) have been an area of intense research and Sarepta’s Elevidys is now the first one to be approved by the US Food and Drug Administration (FDA). million price tag of Elevidys, a one-time gene therapy. Options for managing the symptoms of DMD have been limited.

Pharmaceutical Technology
APRIL 21, 2023
IBM will also provide its knowledge to help Moderna explore the potential use cases of quantum technologies in life sciences. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva. Moderna will join the IBM Quantum Network and the IBM Quantum Accelerator programme.

XTalks
JANUARY 16, 2025
Meanwhile, Medtronics BrainSense Adaptive DBS, newly approved in Europe , could help advance neuromodulation therapy for Parkinsons with real-time brain stimulation adjustments as it becomes available in 2025.

Pharmaceutical Technology
FEBRUARY 3, 2023
With scientists fervently developing mRNA vaccines, nucleic acid therapeutics, and viral vector-based gene therapies, clinicians are set to have a growing number of tools available to treat a wide range of conditions, from infectious diseases to genetic disorders and more. 1] BioNews. Hemophilia News Today. link] [2] Tang Q, et al.
XTalks
NOVEMBER 30, 2022
In this episode, Ayesha shared news about the FDA approval of the first gene therapy for the rare blood disorder Hemophilia B. Read the full articles here: Hemgenix Approved as First Gene Therapy for Hemophilia B. Subscribe to the Xtalks Life Science Podcast to never miss a new episode.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content