NICE recommends gene silencing therapy for porphyria patients on NHS
Pharma Times
OCTOBER 21, 2021
Givlaari uses ‘gene silencing’ RNA interference technology, to target the production of pathogenic compounds in people AHP
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
OCTOBER 21, 2021
Givlaari uses ‘gene silencing’ RNA interference technology, to target the production of pathogenic compounds in people AHP

Bio Pharma Dive
JULY 17, 2023
For $500 million, Novartis will acquire DTx Pharma and its preclinical neurological disease drugs, marking the Swiss company’s latest investment in gene-silencing medicines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JANUARY 31, 2023
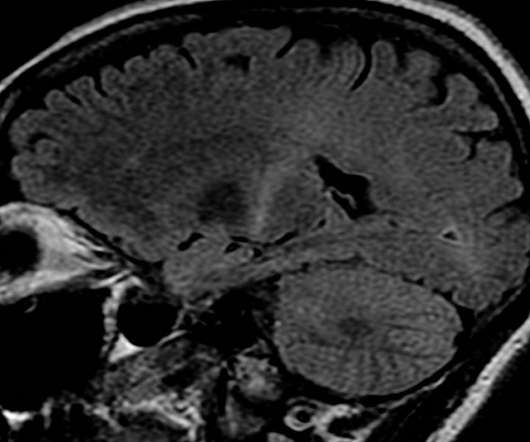
Gene therapy company uniQure has entered into a global licensing agreement with Apic Bio for APB-102 to treat patients with amyotrophic lateral sclerosis (ALS) caused by mutations in superoxide dismutase 1 (SOD1). APB-102 is designed to be a one-time, intrathecally administered gene therapy for ALS patients.
XTalks
OCTOBER 26, 2021
After initial rejection from the National Institute for Health and Care Excellence (NICE) last year, the non-departmental public body of the Department of Health in England has now given the green light to the gene silencing treatment Givlaari (givosiran) for the treatment of the rare metabolic disorder, acute intermittent porphyria (AIP).

pharmaphorum
OCTOBER 13, 2020
UK biotech e-therapeutics has a new CEO, with executive chairman and former Silence Therapeutics chief Ali Mortazavi chosen to spearhead the next stage in the company’s development into gene silencing and other areas. The post New CEO Mortazavi takes UK biotech e-therapeutics into gene silencing appeared first on.

BioPharma Reporter
OCTOBER 2, 2023
Laverock Therapeutics, the gene editing-induced gene silencing platform for human therapeutic applications, has expanded its seed funding round to Â13.5 million.

pharmaphorum
JANUARY 6, 2021
Two years after starting to work together, Novo Nordisk and Dicerna have selected the first candidate from a joint project to find new, gene-silencing drugs for liver-related cardiometabolic diseases. The post Novo Nordisk’s gene silencing alliance with Dicerna bears first fruit appeared first on.

pharmaphorum
AUGUST 27, 2020
Alnylam’s Brendan Martin joined the pharmaphorum podcast for episode 23 to talk about his company’s work in gene-silencing and how it could offer a route to target the current coronavirus pandemic. The post Alnylam, gene-silencing and biotech in 2020: the pharmaphorum podcast appeared first on.
BioSpace
JUNE 16, 2024
The plethora of genes involved in obesity presents an intriguing opportunity for both gene silencing and ex vivo gene therapy approaches.

Pharmaceutical Technology
FEBRUARY 20, 2023
Different approaches that are studied include antisense oligonucleotides (ASOs), and gene therapies, which are in early clinical trials. The therapy is specifically aimed at treating FTD patients who have mutations in their granulin gene, which encodes for the protein progranulin (PGRN). This protein promotes lysosomal function.

BioTech 365
MARCH 10, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: Northwestern U’s novel gene-silencing RNA drug shows promise in glioblastoma.Northwestern U’s novel gene-silencing RNA drug shows promise in glioblastoma aliu Wed, 03/10/2021 – 06:56 from FierceBiotech: Biotech (..)

Scienmag
MAY 9, 2022
and colleagues have described a novel form of gene regulation that is altered in bladder cancer, leading to the boosting of a gene pathway that helps the cancer cells survive during rapid growth. BIRMINGHAM, Ala. Anindya Dutta, MBBS, Ph.D., Credit: UAB BIRMINGHAM, Ala. Anindya Dutta, MBBS, Ph.D.,

Scienmag
JANUARY 4, 2021
BOSTON – A long-running debate over how an important gene-silencing protein identifies its targets has been resolved by researchers at Massachusetts General Hospital (MGH). The findings could yield important implications for development of drugs to treat cancer and other diseases.

Scienmag
JULY 2, 2021
The ongoing effort to explore […].

XTalks
OCTOBER 27, 2021
Ayesha also talked about a new gene silencing treatment for porphyria called Givlaari that received recommendation from England’s NICE after having been initially rejected by the health watchdog last year. Gene Silencing Porphyria Treatment, Givlaari, Finally Wins Over England’s NICE Amid Stellar Long-Term Data.

pharmaphorum
SEPTEMBER 23, 2020
Programmable Oligonucleotide Delivery System (PODS), developed by Sixfold Bioscience, is a versatile system which delivers short interfering RNA (siRNA) gene silencing cargo to specific cancer cells.

Scienmag
OCTOBER 16, 2020
Credit: TMIMS RNA interference is a gene regulatory mechanism in which the expression of specific genes is downregulated by endogenous microRNAs or by small interfering RNAs (siRNAs). Although siRNAs have broad potential for gene-silencing therapy, their instability is one of the difficulties to develop siRNA-based agents.

pharmaphorum
OCTOBER 21, 2021
UK cost-effectiveness watchdog NICE is set to recommend NHS use of Alnylam’s gene-silencing therapy Givlaari in England and Wales for the rare disease acute hepatic porphyria (AHP), after its advisors issued a positive verdict on the drug.
pharmaphorum
JUNE 28, 2021
The reductions matched the efficacy of current therapies for ATTR amyloidosis that require chronic dosing such as Alnylam’s Onpattro (patisiran) and Ionis/Akcea’s Tegsedi (inotersen) – both gene-silencing agents which can cost around $450,000 a year. — Eric Topol (@EricTopol) June 26, 2021.

Scienmag
JUNE 7, 2021
A number of candidate therapies such as CRISPR-Cas9 and gene silencing require the efficient delivery of functional nucleic acids to the cell cytosol and nucleus. Divita Mathur, Research Assistant Professor, is studying cytosolic access and instability of DNA nanoparticles.

pharmaphorum
JULY 28, 2022
Alnylam Pharma has made a name for itself, developing gene-silencing therapies for rare disorders, but its latest discovery could take it into a much larger category – metabolic and cardiovascular disease. Transgenic mice in which the gene has been deleted have improved control of blood glucose and insulin sensitivity.

XTalks
JUNE 14, 2023
Tivanisiran is a preservative-free eye drop that uses RNAi technology, specifically gene silencing, to target and control the signs and symptoms of dry eye disease. It is a small synthetic double-stranded RNA oligonucleotide (siRNA) designed for this purpose, representing the state-of-the-art in gene silencing technology.

pharmaphorum
OCTOBER 19, 2020
If approved, inclisiran would become the first and only gene-silencing drug to reduce low-density lipoprotein cholesterol (LDL-C) in these patients. The drug inhibits PCSK9 – the same target as Amgen’s Repatha (evolocumab) and Sanofi/Regeneron’s Repatha (alirocumab) – but is dosed only twice a year rather than every month.
pharmaphorum
JUNE 1, 2022
The US biotech has just reported phase 2 trial results with the small, interfering RNA (siRNA) gene-silencing drug showing that it an cause a 90% or greater reduction in Lp(a) levels – a risk factor for cardiovascular disease – that was sustained over 48 weeks of follow-up.
pharmaphorum
MARCH 4, 2021
Tivanisiran is based on gene silencing technology using RNA interference (RNAi) and selectively inhibits production of the transient receptor potential cation channel (TRPV1).

pharmaphorum
DECEMBER 13, 2020
Alexion has been working hard to flesh out is pipeline as well, snapping up Achillion Pharma, Syntimmune, Wilson Therapeutics and Portola and forging an alliance with gene-silencing specialist Dicerna focusing on complement diseases.
pharmaphorum
APRIL 13, 2021
It’s the chronic form of the disease that is the main target, with Ionis is working with GSK on an antisense drug that can suppress the hepatitis B virus (HBV), and Roche is working with Dicerna on a rival gene silencing drug.

pharmaphorum
NOVEMBER 18, 2021
Danish drugmaker Novo Nordisk must like what it has seen in its two-year-old alliance with gene-silencing specialist Dicerna Pharma – it has just agreed to acquire the biotech for $3.3 billion in cash.

pharmaphorum
FEBRUARY 18, 2022
With 28 pioneering speakers from large pharma, innovative biotech and KOLs of academia who are ready to discuss the full and comprehensive range of RNAi drugs from discovery to development and beyond, join us in Boston to hear how they address the major challenges facing the industry.
pharmaphorum
JANUARY 6, 2021
Only this morning, Novo Nordisk selected the first candidate from a project with Dicerna to find new gene-silencing drugs to treat liver-related diseases including NASH. A host of other pharma companies including Gilead, Novo Nordisk, Merck & Co are also developing potential NASH drugs.

pharmaphorum
NOVEMBER 24, 2020
The FDA has approved Alnylam’s gene silencing drug Oxlumo, the first treatment for primary hyperoxaluria type 1 (PH1), an ultra-rare and life-threatening genetic disorder.

pharmaphorum
JANUARY 19, 2023
Alnylam’s gene-silencing drug Amvuttra has been recommended as a treatment for hereditary transthyretin-related (ATTR) amyloidosis by NICE, paving the way for the drug to be made available on the NHS in England and Wales.

The Pharma Data
JANUARY 26, 2021
The company’s lead drug candidate is a novel, targeted immuno-oncology gene therapy for the treatment of multiple cancers. The company’s lead drug candidate is a novel, targeted immuno-oncology gene therapy for the treatment of multiple cancers. Scopus BioPharma Inc.

Pharma Marketing Network
DECEMBER 21, 2020
those that modify the expression of an individual’s genes or repair abnormal genes) has entered clinical practice, including 11 RNA therapeutics, 2 in vivo gene therapies, and 2 gene-modified cell therapies. This relinquishes many of the perceived benefits of RNAi therapies that distinguish them from gene therapy (e.g.,

pharmaphorum
JUNE 8, 2022
Zilebesiran (formerly ALN-AGT) is one of a new breed of gene-silencing drugs that are intended to treat common, chronic diseases with infrequent dosing to boost compliance with treatment.

pharmaphorum
OCTOBER 19, 2020
If approved, inclisiran would become the first and only gene-silencing drug to reduce low-density lipoprotein cholesterol (LDL-C) in these patients. The drug inhibits PCSK9 – the same target as Amgen’s Repatha (evolocumab) and Sanofi/Regeneron’s Repatha (alirocumab) – but is dosed only twice a year rather than every month.

The Pharma Data
APRIL 27, 2023
These early results establish the first human translation of Alnylam’s proprietary C16-siRNA conjugate platform for central nervous system (CNS) delivery and are the first clinical demonstration of gene silencing in the human brain using an RNAi therapeutic.

XTalks
MAY 4, 2021
Researchers at the University of California San Francisco (UCSF) and the Whitehead Institute have developed a novel CRISPR-based tool called “CRISPRoff” that can switch off genes in human cells through epigenetic editing without altering the genetic sequence itself. It’s a great tool for controlling gene expression.”. pyogenes dCas9.
XTalks
FEBRUARY 14, 2022
Related: Gene Silencing Porphyria Treatment, Givlaari, Finally Wins Over England’s NICE Amid Stellar Long-Term Data. It is an autosomal recessive disorder, which means that if both parents are carriers of the faulty gene that causes the disease, there is a one in four chance that their child will develop the disease.

pharmaphorum
JULY 13, 2021
In 2019, for example it agreed to work with Dicerna on gene-silencing for liver-related cardio-metabolic disease in a deal that involved a $225 million upfront payment. The post Novo Nordisk bulks up in rare diseases with $1.2bn Prothena deal appeared first on.

pharmaphorum
MAY 11, 2022
billion takeover of gene-silencing specialist Dicerna Pharma last year, another deal aimed at extending its R&D capabilities by bolting on a new platform technology – in this case RNA interference (RNAi) drugs. The move follows Novo Nordisk’s $3.3

pharmaphorum
DECEMBER 23, 2020
2020 also saw some of the first “tumour agnostic” cancer drugs get to market, with Bayer’s Vitravki (larotrectinib) getting funding in the UK for tumours with confirmed neurotrophic tyrosine receptor kinase (NTRK) gene fusions.”. Rare disease progress.

pharmaphorum
OCTOBER 29, 2020
Already a major player in gene therapy, Novartis has swooped on US startup Vedere Bio in a $280 million deal that builds its position in inherited eye diseases that can lead to blindness. Novartis’ big move in the gene therapy market came when it bought AveXis for $8.7 It also gets two preclinical-stage development projects.

XTalks
MAY 4, 2021
Researchers at the University of California San Francisco (UCSF) and the Whitehead Institute have developed a novel CRISPR-based tool called “CRISPRoff” that can switch off genes in human cells without editing the genetic sequence itself. These modifications regulate gene expression without altering the sequence or structure of DNA.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content