Genetic Therapies Show Early Promise in Treating Obesity
BioSpace
JUNE 16, 2024
The plethora of genes involved in obesity presents an intriguing opportunity for both gene silencing and ex vivo gene therapy approaches.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
BioSpace
JUNE 16, 2024
The plethora of genes involved in obesity presents an intriguing opportunity for both gene silencing and ex vivo gene therapy approaches.
Worldwide Clinical Trials
NOVEMBER 27, 2023
Casgevy, the commercial product formerly known as exa-cel, is administered by taking stem cells out of a patient’s bone marrow and editing a gene in the cells in a laboratory, with the modified cells then infused back into the patient after conditioning treatment to prepare the bone marrow. In June 2023, the U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
XTalks
DECEMBER 13, 2023
The US Food and Drug Administration (FDA) has approved the first gene therapies for the treatment of sickle cell disease, approving two on the same day. Both gene therapies are approved for individuals 12 years of age and older with sickle cell disease. It also affects Hispanic Americans, but at a lower prevalence.
Drug Discovery World
AUGUST 7, 2023
The collaboration will work on developing exosome-encapsulated AAV (exoAAV) vectors as a novel gene delivery technology aimed at improving treatments for heart disease. Susmita Sahoo, Associate Professor of Medicine, Cardiology at Icahn Mount Sinai has been exploring the use of exosomes in gene therapy for several years.

XTalks
APRIL 24, 2023
Approximately 72 percent of rare diseases are genetic, and around 70 percent of rare genetic diseases emerge in childhood. Out of over 7,000 rare diseases, only 5 percent (or less) of rare diseases are thought to have approved treatment options, known as “orphan” therapies.
Drug Discovery World
JULY 3, 2024
Circio has announced updated in vivo data that demonstrates a substantial durability advantage of Circio’s circVec technology over conventional mRNA expression. design is performing very well in vivo , and Circio has now validated expression for up to five months. design. “The circVec 2.1

Pharmaceutical Technology
FEBRUARY 23, 2023
Moderna has entered a strategic research and development partnership with ElevateBio’s Life Edit Therapeutics to discover and develop new in-vivo mRNA gene editing therapies. Our novel editing systems have the potential to precisely modify gene targets for both in vivo and ex vivo therapeutic development.”

XTalks
NOVEMBER 3, 2023
After spending almost an entire day deliberating the safety of Vertex Pharmaceuticals’ and CRISPR Therapeutics’ CRISPR-based gene therapy exa-cel for sickle cell disease, a US Food and Drug Administration (FDA) advisory panel appears to be satisfied with what it saw. CRISPR works as genetic scissors to edit parts of the genome.

Drug Discovery World
SEPTEMBER 6, 2022
DDW Editor Reece Armstrong looks at the cell and gene therapy landscape, examining the challenges facing developers and the trends we can expect to see throughout the year. . There’s no doubt that cell and gene therapies present some of the most exciting opportunities for emerging drugs. billion, compared to $19.9

XTalks
OCTOBER 20, 2023
Intellia said NTLA-2001 is the first investigational in vivo CRISPR-based gene editing therapy cleared to enter late-stage clinical development. As an in vivo therapy, it can edit genes inside the body rather than in cells extracted from patients. ATTR amyloidosis is a rare, progressive and fatal disease.

Drug Discovery World
DECEMBER 6, 2023
Gene editing company Eligo Bioscience has announced a successful $30 million Series B funding round, led by Sanofi Ventures. “We We feel this reflects the strong support for our vision and confirms the potential of Eligo to create a novel class of transformative genetic medicines.”

pharmaphorum
JANUARY 29, 2021
Cutting edge’ is, for once, a truly apt description when it comes to gene editing – both because the field is pushing medicine into areas we might never have dreamed possible, and because these technologies involve literally cutting DNA at a specific point in the genome. Zinc fingers. billion in funding.

Drug Discovery World
MAY 17, 2024
From a weight loss drug that prevents heart attacks and a gene therapy that restores hearing, to a vaccine that can treat viruses that don’t exist yet, our chosen news stories this week all represent potential breakthroughs in their respective fields.

The Pharma Data
JANUARY 17, 2021
LONDON–( BUSINESS WIRE )– Ixaka Ltd , an integrated cell and gene therapy company focused on the natural power of the body to cure disease, launches today. The new business will continue to develop Ixaka’s proprietary technologies – concentrated multi-cell therapies (MCTs) and targeted nanoparticle (TNP) therapeutics.

Drug Discovery World
MARCH 7, 2024
Ginkgo Bioworks has acquired Patch Biosciences, with the intention to strengthen its gene therapy services, cell therapy services, and RNA therapeutics services. The collaboration achieved its goals of enhancing the AAV production titres of Biogen’s gene therapy manufacturing processes.

BioPharma Reporter
FEBRUARY 23, 2023
The messenger RNA (mRNA) specialist Moderna has teamed up with ElevateBio-owned Life Edit Therapeutics to develop gene editing therapies that are delivered into patients in vivo.
Scienmag
NOVEMBER 12, 2020
Gong Chen at Jinan University, Guangzhou, China recently reported the first non-human primate study demonstrating successful in vivo neural regeneration from brain internal glial cells for stroke repair. Credit: Jinan University Stroke is a leading cause of death and severe long-term disability with limited treatment available.
Pharmaceutical Technology
MAY 18, 2023
Takeda oncology drug discovery unit head Kathy Seidl stated: “We are encouraged by KSQ’s CRISPRomics platform and its ability to perform in vivo genetic screens for the discovery and validation of tumour targets, which have the potential to modulate the innate and adaptive immune system. “We

pharmaphorum
JANUARY 10, 2022
Bayer has bolstered its cell and gene therapy platform by securing access to a CRISPR-based gene-editing platform developed by US biotech Mammoth Biosciences. CRISPR drugs can be used to modify the expression of disease-associated proteins in the body, for example, by correcting a mutation in a specific gene.

Delveinsight
FEBRUARY 11, 2021
Immune-stimulating antibodies, which target the receptor CD40, are emerging as promising therapies for pancreatic cancer. Immune-stimulating antibodies, which target the receptor CD40, are emerging as promising therapies for pancreatic cancer. Avrobio gene therapy eradicates toxic substrate in Fabry patient.

pharmaphorum
SEPTEMBER 28, 2022
Vertex Pharma and partner CRISPR Therapeutics will start a rolling marketing application in the US for their gene-editing drug for sickle cell disease (SCD) and beta thalassaemia later this year. The time places exa-cel in pole position to become the first drug developed based on CRISPR/Cas9 gene-editing technology to reach the market.

Pharma Marketing Network
DECEMBER 21, 2020
Almost two decades after the human genome was sequenced, a trickle of new genetic medicines (i.e., those that modify the expression of an individual’s genes or repair abnormal genes) has entered clinical practice, including 11 RNA therapeutics, 2 in vivo gene therapies, and 2 gene-modified cell therapies.

pharmaphorum
JUNE 23, 2022
Novartis has shouldered its way into the in vivo gene editing category via a deal with US biotech Precision BioSciences, focused on a therapy for sickle cell disease (SCD). It is also working with Eli Lilly on in vivo gene-editing drugs against three targets, including one in Duchenne muscular dystrophy, under the terms of a $1.4
Pharmaceutical Technology
SEPTEMBER 29, 2022
Scribe Therapeutics and Sanofi have signed a strategic partnership to expedite the development of breakthrough clustered regularly interspaced short palindromic repeats (CRISPR)-based cell therapies for cancer. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Pharmaceutical Technology
AUGUST 17, 2022
These programmes will include therapies and vaccines in infectious disease and oncology areas. oRNA molecules have been demonstrated to possess increased stability in vivo compared to linear mRNA and can potentially create more quantities of therapeutic proteins within the body. .

Drug Discovery World
AUGUST 14, 2023
Lung-I Cheng, Vice President and Head of Cell & Gene Therapy Service Line at AmerisourceBergen, and Cori Gorman, Senior Director of CMC and Regulatory Affairs at Biopharma Excellence, offer advice on navigating the different and sometimes contradictory regulatory requirements in the US and EU. billion in 2020 to $15.5

pharmaphorum
AUGUST 24, 2021
Vertex Pharma has ramped up its involvement in gene-editing medicines for the third time in a matter of months, agreeing a partnership with CRISPR specialist Arbor Biotechnologies that could be worth up to $1.2
Drug Discovery World
JUNE 30, 2023
This pairing includes reprogrammed cells for both wild and mutation-specific Huntington’s Disease (H), Amyotrophic Lateral Sclerosis (ALS), and Alzheimer’s Disease (AD) and is expanding to include custom disease-relevant mutations. Disease relevant in vitro assays improve translation in vivo, leading to clinical success.
Drug Discovery World
OCTOBER 27, 2023
Animal models, Ewart explains, are limited by their genetic and physiological differences from humans. With cues from a diverse population of tissue-specific cell types, extracellular matrix proteins, and biomechanical forces, cells grown in organ-chips closely emulate their in vivo counterparts and replicate tissue-level functions.

WCG Clinical
MAY 28, 2024
Recent headlines have highlighted the potential for Chimeric Antigen Receptor (CAR)-based therapies to provide clinical benefits to persons affected by lupus. An expected and well-known side effect of these B cell-targeted therapies is “B cell aplasia”— i.e. partial or complete depletion of B cells from circulation and immune organs.

Delveinsight
JANUARY 12, 2021
The companies believe that their candidate in its original IgG format has shown potent neutralization activity in in vitro assays and in an in vivo animal model. Bluebird spins off to two companies, cleaving off its gene therapy, and cancer units. Further, Birinapant also complements the anti-tumor activity of the immune system.

Pharmaceutical Technology
MAY 17, 2023
Scribe Therapeutics has entered a strategic collaboration with Eli Lilly and Company subsidiary Prevail Therapeutics for accelerating in vivo CRISPR-based therapies to target the causes of serious neurological and neuromuscular diseases. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.
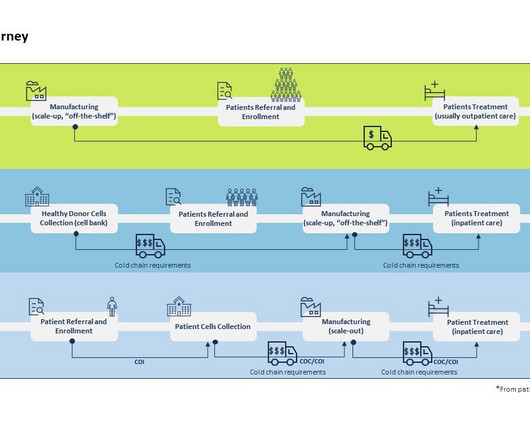
Pharmaceutical Technology
MAY 24, 2023
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.

The Pharma Data
DECEMBER 13, 2020
“Our company and R&D portfolio are entering into an exciting phase, as evidenced by the recent close of Series B financing and submission of the first gene editing product IND in China,” said Dong Wei, Ph.D.?CEO Prior to joining EdiGene, he was Vice President of KLUS Pharma and focused on cell therapy and new technologies.

Drug Discovery World
JULY 5, 2024
The last few days have seen some interesting developments related to gene editing, including the discovery of a new mechanism for genetic programming and evidence in favour of redosing CRISPR-based therapies, as well as significant investment and new indications for gene therapies.

Roots Analysis
JANUARY 14, 2024
Moreover, the exuberant development of biologics has revolutionized the treatment of a range of therapeutic conditions, which has further contributed to the exponential growth in the current demand for biologic therapies developed by biologics manufacturing companies.
Pharmaceutical Technology
AUGUST 18, 2022
The US Food and Drug Administration (FDA) has granted approval for bluebird bio ’s Zynteglo (betibeglogene autotemcel, beti-cel) for the treatment of the underlying genetic cause of beta?thalassemia A custom-made, one-dose gene therapy, Zynteglo is indicated for such patients who need red blood cells (RBCs) transfusions on a regular basis.

The Pharma Data
DECEMBER 27, 2020
PARIS–( BUSINESS WIRE )– Regulatory News: Lysogene (FR0013233475 – LYS) (Paris:LYS), a phase 3 gene therapy platform company targeting central nervous system (CNS) diseases, today reports positive biomarker data from the ongoing AAVance clinical trial with LYS-SAF302 for the treatment of MPS IIIA (NCT03612869).

Drug Discovery World
SEPTEMBER 14, 2023
Also in July 2023, Alexion, AstraZeneca Rare Disease (Alexion) and Pfizer entered into an agreement for Alexion to purchase and license the assets of Pfizer’s early-stage rare disease gene therapy portfolio for a total consideration of up to $1bn, plus tiered royalties on sales. billion, a decrease of $15.0 Vaccines were up 9.1%

Roots Analysis
AUGUST 31, 2023
Overview of Gene Switch The notion that genes might be turned on and off was discovered several decades ago when studies revealed that E. Gene switches are sites on genes where regulatory molecules can bind to trigger transcription process, leading to expression of a particular gene.

Pharmaceutical Technology
JUNE 15, 2023
Verve Therapeutics and Eli Lilly and Company have entered an exclusive research partnership to advance the former’s preclinical stage in vivo gene editing programme targeting lipoprotein(a) (Lp(a)) to treat atherosclerotic cardiovascular disease (ASCVD). The company will receive a combined upfront payment and equity investment of $60m.

Roots Analysis
SEPTEMBER 20, 2023
Cell therapies are based on the premise that the patient’s own cells ( autologous ), or those from a healthy donor ( allogeneic ), can be genetically re-programmed to combat various diseases. In this form of therapy, patients are injected with living and intact human cells that are deemed to be capable of providing therapeutic benefit.

Drug Discovery World
MAY 15, 2024
In vivo proof-of-concept for Circio Holding’s differentiated circVec platform approach to gene therapy has been demonstrated in two posters at the American Society of Gene & Cell Therapy (ASGCT) 2024 annual meeting. In recent experiments, Circio has observed up to four months circVec durability in vivo.

The Pharma Data
DECEMBER 9, 2020
The Chinese biopharmaceutical industry is growing in leaps and bounds, but there is still a huge unmet need when it comes to getting patients access to the breakthrough therapeutic modalities and platforms like RNAi, cell and gene therapy and others. A solution could be at hand with Overland Pharmaceuticals.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content