Zootechnical Animal Food Substances; a New Category of Animal Food Additives Proposed
FDA Law Blog
JUNE 28, 2023
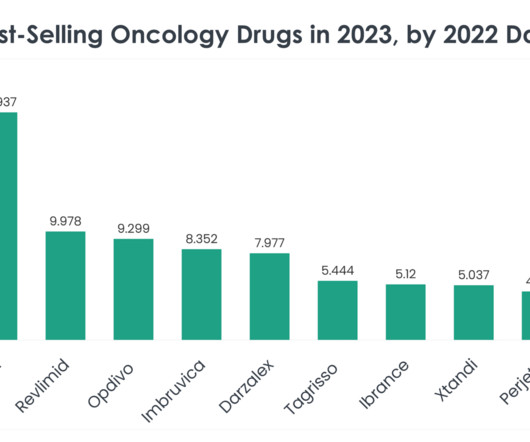
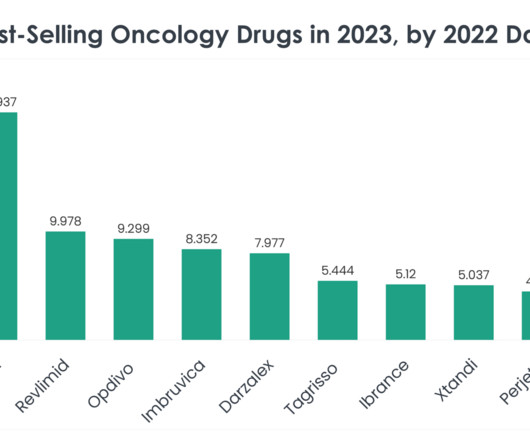
1844 – Animal Drug and Animal Generic Drug User Fee Amendments of 2023. The proposed legislation appears to be an effort to resolve issues created by FDA’s Center for Veterinary Medicine’s (CVM) interpretation of the Federal Food, Drug, and Cosmetic Act (FDC Act) definition of food.













Let's personalize your content