A Short-Term Gain for a Long-Term Loss? The Build Back Better Act’s Medicare Drug Price Negotiation Program Ignores Hatch-Waxman/BPCIA Realities. and that May Mean Big Bad Business for Generic Drug/Biosimilar Manufacturers
FDA Law Blog
NOVEMBER 16, 2021
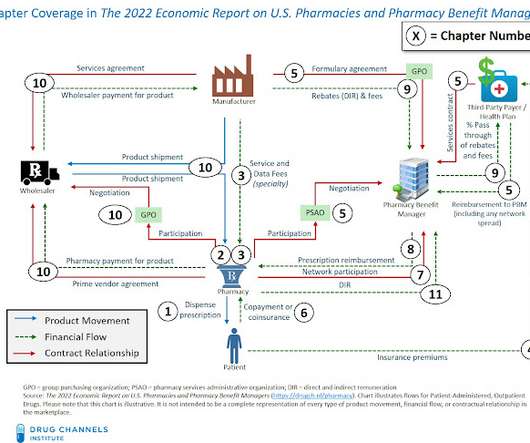
1) publish a list of selected drugs in accordance with section 1192; (2) enter into agreements with manufacturers of selected drugs with respect to such period, in accordance with section 1193; (3) negotiate and, if applicable, renegotiate maximum fair prices for such selected drugs, in accordance with section 1194; and. (4)




























Let's personalize your content